| |
| Names | |
|---|---|
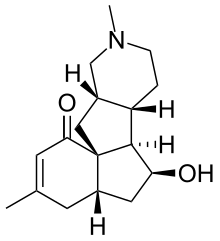
| Preferred IUPAC name
(4aS,6S,6aR,6bS,10aS,11aS)-6-Hydroxy-3,9-dimethyl-4,4a,5,6,6a,6b,7,8,9,10,10a,11-dodecahydro-1H-benzo[3a,4]pentaleno[2,1-c]pyridin-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H25NO2 | |
| Molar mass | 275.392 g·mol−1 |
| Melting point | 165 to 166 °C (329 to 331 °F; 438 to 439 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
(−)-Magellanine is a member of the Lycopodium alkaloid class of natural products. It was isolated from the club moss Lycopodium magellanicum in 1976.[1] It has been synthesized five times, with the first synthesis having been completed by the Larry E. Overman group at the University of California, Irvine in 1993.[2] It has also been synthesized by the Leo Paquette group in 1993 at Ohio State University,[3] the Chun-Chen Liao group in 2002 at National Tsing Hua University,[4] the Miyuki Ishikazi and Tamiko Takahashi groups in 2005 at the Josai International University and Tokyo University of Science,[5] and the Chisato Mukai group in 2007 at the Kanazawa University.[6] One partial synthesis was completed by the A. I. Meyers group in 1995 at Colorado State University.[7]
Biosynthetically, it is thought to have been derived from lysine. This was determined by conducting feeding studies of radiolabeled precursors.[8]
YouTube Encyclopedic
-
1/2Views:76 9875 283
-
Ferdinand Magellan
-
ESOcast 41: Going South — Special 50th anniversary episode #1
Transcription
References
- ^ a b Castillo, Mariano; Loyola, Luis A.; Morales, Glauco; Singh, Ishwar; Calvo, Crispin; Rolland, Herbert L.; MacLean, David B. (1976). "Isolation and Structure". Canadian Journal of Chemistry. 54 (18): 2893–2899. doi:10.1139/v76-409.
- ^ Hirst, Gavin C. (1993). "First total synthesis of Lycopodium alkaloids of the magellanane group. Enantioselective total syntheses of (-)-magellanine and (+)-magellaninone". Journal of the American Chemical Society. 115 (7): 2992–2993. doi:10.1021/ja00060a064.
- ^ Williams, John P. (1994). "Total Synthesis of the Lycopodium Alkaloids Magellanine and Magellaninone by Three-fold Annulation of 2-Cyclopentenone". Journal of the American Chemical Society. 116 (11): 4689–4696. doi:10.1021/ja00090a017.
- ^ Yen, Chi-Feng (2002). "Concise and Efficient Total Synthesis of Lycopodium Alkaloid Magellanine". Angewandte Chemie International Edition. 41 (21): 4090–4093. doi:10.1002/1521-3773(20021104)41:21<4090::AID-ANIE4090>3.0.CO;2-#.
- ^ Ishizaki, Miyuki; Niimi, Yuka; Hoshino, Osamu; Hara, Hiroshi; Takahashi, Tamiko (2005). "A formal total synthesis of Lycopodium alkaloid, (±)-magellanine, by using the intramolecular Pauson Khand reaction". Tetrahedron. 61 (16): 4053–4065. doi:10.1016/j.tet.2005.02.044.
- ^ Kozaka, Takashi (2008). "ChemInform Abstract: Stereoselective Total Synthesis of Three Lycopodium Alkaloids, (-)-Magellanine (I), (+)-Magellaninone (II), and (+)-Paniculatine (III), Based on Two Pauson—Khand Reactions". ChemInform. 39 (18). doi:10.1002/chin.200818182.
- ^ Meyers, A. I. "Partial Synthesis". J. Chem. Soc. Chem. Commun. 1995: 2511–2512.
- ^ Ma, Xiaoqiang; Gang, David R. (2004). "Biosynthesis of the Lycopodium Alkaloids". Nat. Prod. Rep. 21: 752–772. doi:10.1039/b409720n.
