| |
| Names | |
|---|---|
| Other names
Ferric pyrophosphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.030.160 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
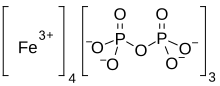
| Fe4(P2O7)3 | |
| Molar mass | 745.224 (anhydrate) 907.348 (nonahydrate) |
| Appearance | yellow solid (nonahydrate)[1] |
| insoluble | |
| Pharmacology | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iron(III) pyrophosphate is an inorganic chemical compound with the formula Fe4(P2O7)3.
YouTube Encyclopedic
-
1/3Views:19 88010 54546 979
-
How to Write the Formula for Iron (III) phosphate
-
Molar Mass / Molecular Weight of Fe3(PO4)2: Iron (III) phosphate
-
How to Write the Formula for Iron (III) Sulfate
Transcription
Synthesis
Anhydrous iron(III) pyrophosphate can be prepared by heating the mixture of iron(III) metaphosphate and iron(III) phosphate under oxygen with the stoichiometric ratio 1:3. The reactants can be prepared by reacting iron(III) nitrate nonahydrate with phosphoric acid.[5]
It can be also prepared via the following reaction:[6]
- 3 Na4P2O7(aq) + 4 FeCl3(aq) → Fe4(P2O7)3(s) + 12 NaCl(aq)
References
- ^ W.M.Haynes. CRC Handbook of Chemistry and Physics (97th edition). New York: CRC Press, 2016. pp 4-68
- ^ "Summary Basis of Decision (SBD) for Triferic Avnu". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ "Triferic Avnu- ferric pyrophosphate citrate solution". DailyMed. 15 September 2020. Retrieved 29 May 2022.
- ^ Elbouaanani, L.K; Malaman, B; Gérardin, R; Ijjaali, M (2002). "Crystal Structure Refinement and Magnetic Properties of Fe4(P2O7)3 Studied by Neutron Diffraction and Mössbauer Techniques". Journal of Solid State Chemistry. 163 (2). Elsevier BV: 412–420. Bibcode:2002JSSCh.163..412E. doi:10.1006/jssc.2001.9415. ISSN 0022-4596.
- ^ Rossi L, Velikov KP, Philipse AP (May 2014). "Colloidal iron(III) pyrophosphate particles". Food Chem. 151: 243–7. doi:10.1016/j.foodchem.2013.11.050. PMID 24423528.
External links
- "Ferric pyrophosphate nonahydrate". Drug Information Portal. U.S. National Library of Medicine.
- "Ferric pyrophosphate citrate". Drug Information Portal. U.S. National Library of Medicine.
