| |||
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChemSpider | |||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C24H14 | |||
| Molar mass | 302.376 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
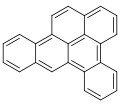
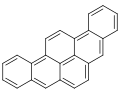
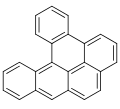
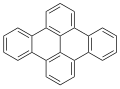
Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
Dibenzopyrenes have been recognized for their suspected human carcinogenicity.[1] The most notable dibenzopyrene isomer, dibenzo[a,l]pyrene is a constituent of tobacco smoke[2] and is thought to be 30 to 100 times more potent as a carcinogen than benzo[a]pyrene.[3][4] The four dibenzopyrene isomers; dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene are included in the list of 16 EU priority polycyclic aromatic hydrocarbons due to their mutagenicity and suspected human carcinogenicity.
Primary sources of dibenzopyrenes in the environment are combustion of wood and coal,[5] gasoline and diesel exhaust,[6] and tires.[7]
YouTube Encyclopedic
-
1/2Views:5371 382
-
Indoor air pollution in developing nations - Video Learning - WizScience.com
-
Tire manufacturing
Transcription
References
- ^ Boström, C. E.; Gerde, P; Hanberg, A; Jernström, B; Johansson, C; Kyrklund, T; Rannug, A; Törnqvist, M; Victorin, K; Westerholm, R (2002). "Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air". Environmental Health Perspectives. 110 Suppl 3: 451–88. doi:10.1289/ehp.110-1241197. PMC 1241197. PMID 12060843.
- ^ Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- ^ "Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures". Integrated Risk Information System (IRIS). United States Environmental Protection Agency. 2010.
- ^ Muller, Pavel (1997). "Scientific criteria document for multimedia standards development, polycyclic aromatic hydrocarbons (PAH)". Standards Development Branch, Ontario Ministry of Environment and Energy.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Masala, Silvia; Bergvall, Christoffer; Westerholm, Roger (15 August 2012). "Determination of benzo[a]pyrene and dibenzopyrenes in a Chinese coal fly ash certified reference material". Science of the Total Environment. 432: 97–102. Bibcode:2012ScTEn.432...97M. doi:10.1016/j.scitotenv.2012.05.081. PMID 22728296.
- ^ Bergvall, Christoffer; Westerholm, Roger (2009). "Determination of highly carcinogenic dibenzopyrene isomers in particulate emissions from two diesel- and two gasoline-fuelled light-duty vehicles". Atmospheric Environment. 43 (25): 3883–3890. Bibcode:2009AtmEn..43.3883B. doi:10.1016/j.atmosenv.2009.04.055.
- ^ Sadiktsis, Ioannis; Bergvall, Christoffer; Johansson, Christer; Westerholm, Roger (2012). "Automobile Tires—A Potential Source of Highly Carcinogenic Dibenzopyrenes to the Environment". Environmental Science & Technology. 46 (6): 3326–3334. Bibcode:2012EnST...46.3326S. doi:10.1021/es204257d. PMID 22352997.