| B lymphocyte cell | |
|---|---|
 Animation of B cell | |
| Details | |
| Precursor | Hematopoietic stem cell |
| System | Immune system |
| Identifiers | |
| Latin | lymphocytus B |
| MeSH | D001402 |
| FMA | 62869 |
| Anatomical terms of microanatomy | |
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype.[1] They function in the humoral immunity component of the adaptive immune system.[1] B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors.[2] When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell.[2] In addition, B cells present antigens (they are also classified as professional antigen-presenting cells, APCs) and secrete cytokines.[1] In mammals, including marsupials [3] B cells mature in the bone marrow, which is at the core of most bones.[4] In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick,[5] which is why the B stands for bursa and not bone marrow, as commonly believed.
B cells, unlike the other two classes of lymphocytes, T cells and natural killer cells, express B cell receptors (BCRs) on their cell membrane.[1] BCRs allow the B cell to bind to a foreign antigen, against which it will initiate an antibody response.[1] B cell receptors are extremely specific, with all BCRs on a B cell recognizing the same epitope.[6]
YouTube Encyclopedic
-
1/5Views:1 573 594184 70924 953543 40871 184
-
B lymphocytes (B cells) | Immune system physiology | NCLEX-RN | Khan Academy
-
B cell Activation and Differentiation (PART 1): T Independent Activation (FL-Immuno/48)
-
B-Cell Activation and Class Switching | Immunology | Physiology Series
-
Immunology - Adaptive Immunity (B cell Activation, Hypermutation and Class Switching Overview)
-
B-Cell Development in the Bone Marrow – Lymphocyte Development | Lecturio
Transcription
Let's just talk about the humoral response right now, that deals with B lymphocytes. So B lymphocytes or B cells-- let me do them in blue. So let's say that that is a B lymphocyte. It's a subset of white blood cells called lymphocytes. It comes from the bone marrow and that's where the-- well, the B comes from bursa of Fabricius, but we don't want to go into detail there. But they have all of these proteins on their surface. Actually, close to 10,000 of them. I get very excited about B cells and I'll tell you why in a second. It has all of these proteins on them that look something like this. I'll just draw a couple of them. These are actually protein complexes, you can kind of view them. They actually have four separate proteins on them and we can call these proteins membrane bound antibodies. And I'll talk a lot more about antibodies. You've probably heard the word. You have antibodies for such and such flu, or such and such virus, and we're going to talk more about that in the future, but antibodies are just proteins. They're often referred to as immunoglobulins. These are essentially equivalent words. Antibodies or immunoglobulins-- and they're really just proteins. Now, B cells have these on the surface of their membranes. These are membrane bound. Usually when people talk about antibodies, they're talking about free antibodies that are going to just be floating around like that. And I'm going to go into more detail on how those are produced. Now what's really, really, really, really, really interesting about these membrane bound antibodies and these B cells in particular is that a B cell has one type of membrane bound antibody on it . It's going to also have antibodies, but those antibodies are going to be different. So we'll focus on where they're different. Let me just draw them the same color first and then we'll focus on where they're different. These are both B cells. They both have these antibodies on them. The interesting thing is that from one B cell to another B cell, they have a variable part on this antibody that could take on a bunch of different forms. So this one might look like that and that. So these long-- I'll go into more detail on that. The fixed portion, you can imagine is green for any kind of antibody, and then there's a variable portion. So maybe this guy's variable portion is-- I'll do it in pink. And every one of the antibodies bound to his membrane are going to have that same variable portion. This different B cell is going to have different variable portions. So I'll do that in a different color. Maybe I'll do it in magenta. So his variable portions are going to be different. Now he has 10,000 of these on a surface and every one of these have the same variable portions, but they're all different from the variable portions on this B cell. There's actually 10 billion different combinations of variable portions. So the first question-- and I haven't even told you what the variable portions are good for-- is, how do that many different combinations arise? Obviously these proteins-- or maybe not so obviously-- all these proteins that are part of most cells are produced by the genes of that cell. So if I draw-- this is the nucleus. It's got DNA inside the nucleus. This guy has a nucleus. It's got DNA inside the nucleus. If these guys are both B cells and they're both coming from the same germ line, they're coming from the same, I guess, ancestry of cells, shouldn't they have the same DNA? If they do have the same DNA, why are the proteins that they're constructing different? How do they change? And this is why I find B cells-- and you'll see this is also true of T cells-- to be fascinating is, in their development, in their hematopoiesis-- that's just the development of these lymphocytes. At one stage in their development, there's just a lot of shuffling of the portion of their DNA that codes for here, for these parts of the protein. There's just a lot of shuffling that occurs. Most of when we talk about DNA, we really want to preserve the information, not have a lot of shuffling. But when these lymphocytes, when these B cells are maturing, at one stage of their maturation or their development, there's intentional reshuffling of the DNA that codes for this part and this part. And that's what leads to all of the diversity in the variable portions on these membrane bound immunoglobulins. And we're about to find out why there's that diversity. So there's tons of stuff that can infect your body. Viruses are are mutating and evolving and so are bacteria. You don't know what's going to enter your body. So what the immune system has done through B cells-- and we'll also see it through T cells-- it says, hey, let me just make a bunch of combinations of these things that can essentially bind to whatever I get to. So let's say that there's just some new virus that shows up, right? The world has never seen this virus before this B cell, it'll bump into this virus and this virus won't attach. Another B cell will bump into this virus and it won't attach. And maybe several thousands of B cells will bump into this virus and it won't attach, but since I have so many B cells having so many different combinations of these variable portions on these receptors, eventually one of these B cells is going to bond. Maybe it's this one. He's going to bond to part of the surface of this virus. It could also be to part of a surface of a new bacteria, or part of a surface for some foreign protein. And part of the surface that it binds on the bacteria-- so maybe it binds on that part of the bacteria-- this is called an epitope. So once this guy binds to some foreign pathogen-- and remember, the other B cells won't-- only the particular one that had the particular combination, one of the 10 to the 10th. And actually, there aren't 10 to the 10th combinations. During their development, they weed out all of the combinations that would bind to things that are essentially you, that there shouldn't be an immune response to. So we could say self-responding combinations weeded out. So there actually aren't 10 to the 10th, 10 billion combinations of these-- something smaller than that. You have to take out all the combinations that would have bound to your own cells, but there's still a super huge number of combinations that are very likely to bond, at least to some part of some pathogen of some virus or some bacteria. And as soon as one of these B cells binds, it says, hey guys, I'm the lucky guy who happens to fit exactly this brand new pathogen. He becomes activated after binding to the new pathogen. And I'm going to go into more detail in the future. In order to really become activated, you normally need help from helper T cells, but I don't want to confuse you in the video. So in this case, I'm going to assume that activation can only occur-- or that it just needs to respond, it just needs to essentially be triggered by binding with the pathogen. In most cases, you actually need the helper T cells as well. And we'll discuss why that's important. It's kind of a fail safe mechanism for your immune system. But once this guy gets activated, he's going to start cloning himself. He's going to say, look, I'm the guy that can match this virus here-- and so he's going to start cloning himself. He's going to start dividing and repeating himself. So there's just going to be multiple versions of this guy. So they all start to replicate and they also differentiate-- differentiate means they start taking particular roles. So there's two forms of differentiation. So many, many, many hundreds or thousands of these are going to be produced. And then some are going to become memory cells, which are essentially just B cells that stick around a long time with the perfect receptor on them, with the perfect variable portion of their receptor on them. So some will be memory cells and they're going to be in higher quantities than they were originally. So if if this guy invades our bodies 10 years in the future, they're going to have more of these guys around that are more likely to bump into them and start and get activated and then some of them are going to turn into effector cells. And effector cells are generally cells that actually do something. What the effector cells do is, they turn into antibody-- they turn into these effector B cells-- or sometimes they're called plasma cells. They're going to turn into antibody factories. And the antibodies they're going to produce are exactly this combination, the date that they originally had being membrane bound. So they're just going to start producing these antibodies that we talk about with the exact-- they're going to start spitting out these antibodies. They're going to start spitting out tons and tons of these proteins that are uniquely able to bind to the new pathogen, this new thing in question. So an activated effector cell will actually produce 2,000 antibodies a second. So you can imagine, if you have a lot of these, you're going to have all of a sudden a lot of antibodies floating around in your body and going into the body tissues. And the value of that and why this is the humoral system is, all of a sudden, you have all of these viruses that are infecting your system, but now you're producing all of these antibodies. The effector cells are these factories and so these specific antibodies will start bonding. So let me draw it like this. The specific antibodies will start bonding to these viruses and that has a couple of values to it. One is, it essentially tags them for pick up. Now phagocytosis-- this is called opsonization. When you tag molecules for pickup and you make them easier for phagocytes to eat them up, this is what-- antibodies are attaching and say, hey phagocytes, this is going to make it easier. You should pick up these guys in particular. It also might make these viruses hard to function. I have this big thing hanging off the side of it. It might be harder for them to infiltrate cells and the other thing is, on each of these antibodies you have two identical heavy chains and then two identical light chains. And then they have a very specific variable portion on each one and each of these branches can bond to the epitope on a virus. So you can imagine, what happens if this guy bonds to one epitope and this guy bonds to another virus? Then all of a sudden, these viruses are kind of glued together and that's even more efficient. They're not going to be able to do what they normally do. They're not going to be able to enter cell membranes and they're perfectly tagged. They've been opsonized so that phagocytes can come and eat them up. So we'll talk more about B cells in the future, but I just find it fascinating that there are that many combinations and they have enough combinations to really recognize almost anything that can exist in the fluids of our body, but we haven't solved all of the problems yet. We haven't solved the problem of what happens when things actually infiltrate cells or we have cancer cells? How do we kill cells that have clearly gone astray?
Development


B cells develop from hematopoietic stem cells (HSCs) that originate from bone marrow.[7][8] HSCs first differentiate into multipotent progenitor (MPP) cells, then common lymphoid progenitor (CLP) cells.[8] From here, their development into B cells occurs in several stages (shown in image to the right), each marked by various gene expression patterns and immunoglobulin H chain and L chain gene loci arrangements, the latter due to B cells undergoing V(D)J recombination as they develop.[9]
B cells undergo two types of selection while developing in the bone marrow to ensure proper development, both involving B cell receptors (BCR) on the surface of the cell. Positive selection occurs through antigen-independent signalling involving both the pre-BCR and the BCR.[10][11] If these receptors do not bind to their ligand, B cells do not receive the proper signals and cease to develop.[10][11] Negative selection occurs through the binding of self-antigen with the BCR; if the BCR can bind strongly to self-antigen, then the B cell undergoes one of four fates: clonal deletion, receptor editing, anergy, or ignorance (B cell ignores signal and continues development).[11] This negative selection process leads to a state of central tolerance, in which the mature B cells do not bind self antigens present in the bone marrow.[9]
To complete development, immature B cells migrate from the bone marrow into the spleen as transitional B cells, passing through two transitional stages: T1 and T2.[12] Throughout their migration to the spleen and after spleen entry, they are considered T1 B cells.[13] Within the spleen, T1 B cells transition to T2 B cells.[13] T2 B cells differentiate into either follicular (FO) B cells or marginal zone (MZ) B cells depending on signals received through the BCR and other receptors.[14] Once differentiated, they are now considered mature B cells, or naive B cells.[13]
Activation

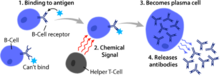
B cell activation occurs in the secondary lymphoid organs (SLOs), such as the spleen and lymph nodes.[1] After B cells mature in the bone marrow, they migrate through the blood to SLOs, which receive a constant supply of antigen through circulating lymph.[15] At the SLO, B cell activation begins when the B cell binds to an antigen via its BCR.[16] Although the events taking place immediately after activation have yet to be completely determined, it is believed that B cells are activated in accordance with the kinetic segregation model [citation needed], initially determined in T lymphocytes. This model denotes that before antigen stimulation, receptors diffuse through the membrane coming into contact with Lck and CD45 in equal frequency, rendering a net equilibrium of phosphorylation and non-phosphorylation. It is only when the cell comes in contact with an antigen presenting cell that the larger CD45 is displaced due to the close distance between the two membranes. This allows for net phosphorylation of the BCR and the initiation of the signal transduction pathway[citation needed]. Of the three B cell subsets, FO B cells preferentially undergo T cell-dependent activation while MZ B cells and B1 B cells preferentially undergo T cell-independent activation.[17]
B cell activation is enhanced through the activity of CD21, a surface receptor in complex with surface proteins CD19 and CD81 (all three are collectively known as the B cell coreceptor complex).[18] When a BCR binds an antigen tagged with a fragment of the C3 complement protein, CD21 binds the C3 fragment, co-ligates with the bound BCR, and signals are transduced through CD19 and CD81 to lower the activation threshold of the cell.[19]
T cell-dependent activation
Antigens that activate B cells with the help of T-cell are known as T cell-dependent (TD) antigens and include foreign proteins.[1] They are named as such because they are unable to induce a humoral response in organisms that lack T cells.[1] B cell responses to these antigens takes multiple days, though antibodies generated have a higher affinity and are more functionally versatile than those generated from T cell-independent activation.[1]
Once a BCR binds a TD antigen, the antigen is taken up into the B cell through receptor-mediated endocytosis, degraded, and presented to T cells as peptide pieces in complex with MHC-II molecules on the cell membrane.[20] T helper (TH) cells, typically follicular T helper (TFH) cells recognize and bind these MHC-II-peptide complexes through their T cell receptor (TCR).[21] Following TCR-MHC-II-peptide binding, T cells express the surface protein CD40L as well as cytokines such as IL-4 and IL-21.[21] CD40L serves as a necessary co-stimulatory factor for B cell activation by binding the B cell surface receptor CD40, which promotes B cell proliferation, immunoglobulin class switching, and somatic hypermutation as well as sustains T cell growth and differentiation.[1] T cell-derived cytokines bound by B cell cytokine receptors also promote B cell proliferation, immunoglobulin class switching, and somatic hypermutation as well as guide differentiation.[21] After B cells receive these signals, they are considered activated.[21]

Once activated, B cells participate in a two-step differentiation process that yields both short-lived plasmablasts for immediate protection and long-lived plasma cells and memory B cells for persistent protection.[17] The first step, known as the extrafollicular response, occurs outside lymphoid follicles but still in the SLO.[17] During this step activated B cells proliferate, may undergo immunoglobulin class switching, and differentiate into plasmablasts that produce early, weak antibodies mostly of class IgM.[22]

The second step consists of activated B cells entering a lymphoid follicle and forming a germinal center (GC), which is a specialized microenvironment where B cells undergo extensive proliferation, immunoglobulin class switching, and affinity maturation directed by somatic hypermutation.[23] These processes are facilitated by TFH cells within the GC and generate both high-affinity memory B cells and long-lived plasma cells.[17] Resultant plasma cells secrete large amounts of antibody and either stay within the SLO or, more preferentially, migrate to bone marrow.[23]
T cell-independent activation
Antigens that activate B cells without T cell help are known as T cell-independent (TI) antigens[1] and include foreign polysaccharides and unmethylated CpG DNA.[17] They are named as such because they are able to induce a humoral response in organisms that lack T cells.[1] B cell response to these antigens is rapid, though antibodies generated tend to have lower affinity and are less functionally versatile than those generated from T cell-dependent activation.[1]
As with TD antigens, B cells activated by TI antigens need additional signals to complete activation, but instead of receiving them from T cells, they are provided either by recognition and binding of a common microbial constituent to toll-like receptors (TLRs) or by extensive crosslinking of BCRs to repeated epitopes on a bacterial cell.[1] B cells activated by TI antigens go on to proliferate outside lymphoid follicles but still in SLOs (GCs do not form), possibly undergo immunoglobulin class switching, and differentiate into short-lived plasmablasts that produce early, weak antibodies mostly of class IgM, but also some populations of long-lived plasma cells.[24]
Memory B cell activation
Memory B cell activation begins with the detection and binding of their target antigen, which is shared by their parent B cell.[25] Some memory B cells can be activated without T cell help, such as certain virus-specific memory B cells, but others need T cell help.[26] Upon antigen binding, the memory B cell takes up the antigen through receptor-mediated endocytosis, degrades it, and presents it to T cells as peptide pieces in complex with MHC-II molecules on the cell membrane.[25] Memory T helper (TH) cells, typically memory follicular T helper (TFH) cells, that were derived from T cells activated with the same antigen recognize and bind these MHC-II-peptide complexes through their TCR.[25] Following TCR-MHC-II-peptide binding and the relay of other signals from the memory TFH cell, the memory B cell is activated and differentiates either into plasmablasts and plasma cells via an extrafollicular response or enter a germinal center reaction where they generate plasma cells and more memory B cells.[25][26] It is unclear whether the memory B cells undergo further affinity maturation within these secondary GCs.[25] In vitro activation of memory B cells can be achieved through stimulation with various activators, such as pokeweed mitogen or anti-CD40 monoclonal antibodies, however, a study found a combination of R-848 and recombinant human IL-2 to be the most efficient activator.[27]
B cell types

- Plasmablast
- A short-lived, proliferating antibody-secreting cell arising from B cell differentiation.[1] Plasmablasts are generated early in an infection and their antibodies tend to have a weaker affinity towards their target antigen compared to plasma cell.[17] Plasmablasts can result from T cell-independent activation of B cells or the extrafollicular response from T cell-dependent activation of B cells.[1]
- Plasma cell
- A long-lived, non-proliferating antibody-secreting cell arising from B cell differentiation.[1] There is evidence that B cells first differentiate into a plasmablast-like cell, then differentiate into a plasma cell.[17] Plasma cells are generated later in an infection and, compared to plasmablasts, have antibodies with a higher affinity towards their target antigen due to affinity maturation in the germinal center (GC) and produce more antibodies.[17] Plasma cells typically result from the germinal center reaction from T cell-dependent activation of B cells, though they can also result from T cell-independent activation of B cells.[24]
- Lymphoplasmacytoid cell
- A cell with a mixture of B lymphocyte and plasma cell morphological features that is thought to be closely related to or a subtype of plasma cells. This cell type is found in pre-malignant and malignant plasma cell dyscrasias that are associated with the secretion of IgM monoclonal proteins; these dyscrasias include IgM monoclonal gammopathy of undetermined significance and Waldenström's macroglobulinemia.[28]
- Memory B cell
- Dormant B cell arising from B cell differentiation.[1] Their function is to circulate through the body and initiate a stronger, more rapid antibody response (known as the anamnestic secondary antibody response) if they detect the antigen that had activated their parent B cell (memory B cells and their parent B cells share the same BCR, thus they detect the same antigen).[26] Memory B cells can be generated from T cell-dependent activation through both the extrafollicular response and the germinal center reaction as well as from T cell-independent activation of B1 cells.[26]
- B-2 cell
- FO B cells and MZ B cells.[29]
- Follicular (FO) B cell (also known as a B-2 cell)
- Most common type of B cell and, when not circulating through the blood, is found mainly in the lymphoid follicles of secondary lymphoid organs (SLOs).[17] They are responsible for generating the majority of high-affinity antibodies during an infection.[1]
- Marginal-zone (MZ) B cell
- Found mainly in the marginal zone of the spleen and serves as a first line of defense against blood-borne pathogens, as the marginal zone receives large amounts of blood from the general circulation.[30] They can undergo both T cell-independent and T cell-dependent activation, but preferentially undergo T cell-independent activation.[17]
- B-1 cell
- Arises from a developmental pathway different from FO B cells and MZ B cells.[29] In mice, they predominantly populate the peritoneal cavity and pleural cavity, generate natural antibodies (antibodies produced without infection), defend against mucosal pathogens, and primarily exhibit T cell-independent activation.[29] A true homologue of mouse B-1 cells has not been discovered in humans, though various cell populations similar to B-1 cells have been described.[29]
- Regulatory B (Breg) cell
- An immunosuppressive B cell type that stops the expansion of pathogenic, pro-inflammatory lymphocytes through the secretion of IL-10, IL-35, and TGF-β.[31] Also, it promotes the generation of regulatory T (Treg) cells by directly interacting with T cells to skew their differentiation towards Tregs.[31] No common Breg cell identity has been described and many Breg cell subsets sharing regulatory functions have been found in both mice and humans.[31] It is currently unknown if Breg cell subsets are developmentally linked and how exactly differentiation into a Breg cell occurs.[31] There is evidence showing that nearly all B cell types can differentiate into a Breg cell through mechanisms involving inflammatory signals and BCR recognition.[31]
Autoimmune disease can result from abnormal B cell recognition of self-antigens followed by the production of autoantibodies.[32] Autoimmune diseases where disease activity is correlated with B cell activity include scleroderma, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, post-infectious IBS, and rheumatoid arthritis.[32]
Malignant transformation of B cells and their precursors can cause a host of cancers, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), hairy cell leukemia, follicular lymphoma, non-Hodgkin's lymphoma, Hodgkin's lymphoma, and plasma cell malignancies such as multiple myeloma, Waldenström's macroglobulinemia, and certain forms of amyloidosis.[33][34]
Abnormal B cells may be relatively large and some diseases include this in their names, such as diffuse large B-cell lymphomas (DLBCLs) and intravascular large B-cell lymphoma.
Patients with B cell alymphocytosis are predisposed to infections.[35]
Epigenetics
A study that investigated the methylome of B cells along their differentiation cycle, using whole-genome bisulfite sequencing (WGBS), showed that there is a hypomethylation from the earliest stages to the most differentiated stages. The largest methylation difference is between the stages of germinal center B cells and memory B cells. Furthermore, this study showed that there is a similarity between B cell tumors and long-lived B cells in their DNA methylation signatures.[36]
See also
References
- ^ a b c d e f g h i j k l m n o p q r s Murphy K (2012). Janeway's Immunobiology (8th ed.). New York: Garland Science. ISBN 9780815342434.
- ^ a b Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "B Cells and Antibodies". Molecular Biology of the Cell (4th ed.). Garland Science.
- ^ Schraven, Andrea L.; Hansen, Victoria L.; Morrissey, K. A.; Stannard, Hayley J.; Ong, Oselyne O. T. W.; Douek, D. C.; Miller, Robert D.; Old, Julie M. (2021). "Single-cell transcriptome analysis of the B-cell repertoire reveals the usage of immunoglobulins in the gray short-tailed opossum (Monodelphis domestica)". Developmental and Comparative Immunology. 123: 104141. doi:10.1016/j.dci.2021.104141.
- ^ Cooper MD (March 2015). "The early history of B cells". Nature Reviews. Immunology. 15 (3): 191–197. doi:10.1038/nri3801. PMID 25656707.
- ^ Glick, Bruce; Chang, Timothy S.; Jaap, R. George (1956-01-01). "The Bursa of Fabricius and Antibody Production". Poultry Science. 35 (1): 224–225. doi:10.3382/ps.0350224. ISSN 0032-5791.
- ^ Jespersen, Martin Closter; Mahajan, Swapnil; Peters, Bjoern; Nielsen, Morten; Marcatili, Paolo (2019). "Antibody Specific B-Cell Epitope Predictions: Leveraging Information From Antibody-Antigen Protein Complexes". Frontiers in Immunology. 10: 298. doi:10.3389/fimmu.2019.00298. PMC 6399414. PMID 30863406.
- ^ Fischer U, Yang JJ, Ikawa T, Hein D, Vicente-Dueñas C, Borkhardt A, Sánchez-García I (November 2020). "Cell Fate Decisions: The Role of Transcription Factors in Early B-cell Development and Leukemia". Blood Cancer Discovery. 1 (3): 224–233. doi:10.1158/2643-3230.BCD-20-0011. PMC 7774874. PMID 33392513.
- ^ a b Kondo M (November 2010). "Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors". Immunological Reviews. 238 (1): 37–46. doi:10.1111/j.1600-065X.2010.00963.x. PMC 2975965. PMID 20969583.
- ^ a b Pelanda R, Torres RM (April 2012). "Central B-cell tolerance: where selection begins". Cold Spring Harbor Perspectives in Biology. 4 (4): a007146. doi:10.1101/cshperspect.a007146. PMC 3312675. PMID 22378602.
- ^ a b Mårtensson IL, Almqvist N, Grimsholm O, Bernardi AI (June 2010). "The pre-B cell receptor checkpoint". FEBS Letters. 584 (12): 2572–2579. doi:10.1016/j.febslet.2010.04.057. PMID 20420836. S2CID 43158480.
- ^ a b c LeBien TW, Tedder TF (September 2008). "B lymphocytes: how they develop and function". Blood. 112 (5): 1570–1580. doi:10.1182/blood-2008-02-078071. PMC 2518873. PMID 18725575.
- ^ Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, et al. (July 1999). "B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals". The Journal of Experimental Medicine. 190 (1): 75–89. doi:10.1084/jem.190.1.75. PMC 2195560. PMID 10429672.
- ^ a b c Chung JB, Silverman M, Monroe JG (June 2003). "Transitional B cells: step by step towards immune competence". Trends in Immunology. 24 (6): 343–349. doi:10.1016/S1471-4906(03)00119-4. PMID 12810111.
- ^ Cerutti A, Cols M, Puga I (February 2013). "Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes". Nature Reviews. Immunology. 13 (2): 118–132. doi:10.1038/nri3383. PMC 3652659. PMID 23348416.
- ^ Harwood NE, Batista FD (2010-01-01). "Early events in B cell activation". Annual Review of Immunology. 28 (1): 185–210. doi:10.1146/annurev-immunol-030409-101216. PMID 20192804.
- ^ Yuseff MI, Pierobon P, Reversat A, Lennon-Duménil AM (July 2013). "How B cells capture, process and present antigens: a crucial role for cell polarity". Nature Reviews. Immunology. 13 (7): 475–486. doi:10.1038/nri3469. PMID 23797063. S2CID 24791216.
- ^ a b c d e f g h i j Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM (March 2015). "The generation of antibody-secreting plasma cells". Nature Reviews. Immunology. 15 (3): 160–171. doi:10.1038/nri3795. PMID 25698678. S2CID 9769697.
- ^ Asokan R, Banda NK, Szakonyi G, Chen XS, Holers VM (January 2013). "Human complement receptor 2 (CR2/CD21) as a receptor for DNA: implications for its roles in the immune response and the pathogenesis of systemic lupus erythematosus (SLE)". Molecular Immunology. 53 (1–2): 99–110. doi:10.1016/j.molimm.2012.07.002. PMC 3439536. PMID 22885687.
- ^ Zabel MD, Weis JH (March 2001). "Cell-specific regulation of the CD21 gene". International Immunopharmacology. Unraveling Mechanisms and Discovering Novel Roles for Complement. 1 (3): 483–493. doi:10.1016/S1567-5769(00)00046-1. PMID 11367532.
- ^ Blum JS, Wearsch PA, Cresswell P (2013-01-01). "Pathways of antigen processing". Annual Review of Immunology. 31 (1): 443–473. doi:10.1146/annurev-immunol-032712-095910. PMC 4026165. PMID 23298205.
- ^ a b c d Crotty S (March 2015). "A brief history of T cell help to B cells". Nature Reviews. Immunology. 15 (3): 185–189. doi:10.1038/nri3803. PMC 4414089. PMID 25677493.
- ^ MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, et al. (August 2003). "Extrafollicular antibody responses". Immunological Reviews. 194: 8–18. doi:10.1034/j.1600-065x.2003.00058.x. PMID 12846803. S2CID 2455541.
- ^ a b Shlomchik MJ, Weisel F (May 2012). "Germinal center selection and the development of memory B and plasma cells". Immunological Reviews. 247 (1): 52–63. doi:10.1111/j.1600-065X.2012.01124.x. PMID 22500831. S2CID 5362003.
- ^ a b Bortnick A, Chernova I, Quinn WJ, Mugnier M, Cancro MP, Allman D (June 2012). "Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens". Journal of Immunology. 188 (11): 5389–5396. doi:10.4049/jimmunol.1102808. PMC 4341991. PMID 22529295.
- ^ a b c d e McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L (December 2011). "Molecular programming of B cell memory". Nature Reviews. Immunology. 12 (1): 24–34. doi:10.1038/nri3128. PMC 3947622. PMID 22158414.
- ^ a b c d Kurosaki T, Kometani K, Ise W (March 2015). "Memory B cells". Nature Reviews. Immunology. 15 (3): 149–159. doi:10.1038/nri3802. PMID 25677494. S2CID 20825732.
- ^ Jahnmatz, Maja; Kesa, Gun; Netterlid, Eva; Buisman, Anne-Marie; Thorstensson, Rigmor; Ahlborg, Niklas (2013-05-31). "Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses". Journal of Immunological Methods. 391 (1): 50–59. doi:10.1016/j.jim.2013.02.009. ISSN 0022-1759. PMID 23454005.
- ^ Ribourtout B, Zandecki M (June 2015). "Plasma cell morphology in multiple myeloma and related disorders". Morphologie. 99 (325): 38–62. doi:10.1016/j.morpho.2015.02.001. PMID 25899140. S2CID 1508656.
- ^ a b c d Baumgarth N (January 2011). "The double life of a B-1 cell: self-reactivity selects for protective effector functions". Nature Reviews. Immunology. 11 (1): 34–46. doi:10.1038/nri2901. PMID 21151033. S2CID 23355423.
- ^ Pillai S, Cariappa A, Moran ST (2005-01-01). "Marginal zone B cells". Annual Review of Immunology. 23 (1): 161–196. doi:10.1146/annurev.immunol.23.021704.115728. PMID 15771569.
- ^ a b c d e Rosser EC, Mauri C (April 2015). "Regulatory B cells: origin, phenotype, and function". Immunity. 42 (4): 607–612. doi:10.1016/j.immuni.2015.04.005. PMID 25902480.
- ^ a b Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF (June 2008). "B-lymphocyte contributions to human autoimmune disease". Immunological Reviews. 223 (1): 284–299. doi:10.1111/j.1600-065X.2008.00646.x. PMID 18613843. S2CID 11593298.
- ^ Shaffer AL, Young RM, Staudt LM (2012-01-01). "Pathogenesis of human B cell lymphomas". Annual Review of Immunology. 30 (1): 565–610. doi:10.1146/annurev-immunol-020711-075027. PMC 7478144. PMID 22224767.
- ^ Castillo JJ (December 2016). "Plasma Cell Disorders". Primary Care. 43 (4): 677–691. doi:10.1016/j.pop.2016.07.002. PMID 27866585.
- ^ Grammatikos Alexandros, Donati Matthew, Johnston Sarah L., Gompels Mark M. Peripheral B Cell Deficiency and Predisposition to Viral Infections: The Paradigm of Immune Deficiencies. Frontiers in Immunology (12)2021 https://www.frontiersin.org/articles/10.3389/fimmu.2021.731643 DOI=10.3389/fimmu.2021.731643
- ^ Kulis M, Merkel A, Heath S, Queirós AC, Schuyler RP, Castellano G, et al. (July 2015). "Whole-genome fingerprint of the DNA methylome during human B cell differentiation". Nature Genetics. 47 (7): 746–756. doi:10.1038/ng.3291. PMC 5444519. PMID 26053498.
