| |
| Names | |
|---|---|
| IUPAC name
Ammonium propanoate
| |
| Other names
Ammonium propionate
propanoic acid, ammonium salt(1:1) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.715 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO2 | |
| Molar mass | 91.110 g·mol−1 |
| Melting point | 45 °C (113 °F; 318 K) |
| Boiling point | 141.7 °C (287.1 °F; 414.8 K) |
| 1 g/mL | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
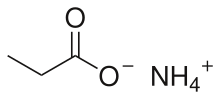
Ammonium propionate or ammonium propanoate is the ammonium salt of propionic acid. It has the chemical formula NH4(C2H5COO).
Reaction
It is formed by the reaction of propionic acid and ammonia.
Uses
It is used in several products, which include: fertilizers, water treatment chemicals, and plant protection products. It is also used in different areas, such as: manufacturing, forestry, agriculture, and fishing.[1]
It also serves as an antiseptic, antifungal agent, antimould agent, and preservative in feed industry or food industry.[2]
Ammonium propionate also prevents spoilage of cosmetics by preventing bacterial growth.[3]
See also
References
- ^ "Ammonium propionate - Substance Information - ECHA". echa.europa.eu. Retrieved 2021-02-22.
- ^ "Ammonium Propionate Properties, Molecular Formula, Applications - WorldOfChemicals". www.worldofchemicals.com. Archived from the original on 2022-02-17. Retrieved 2021-02-22.
- ^ "Ammonium Propionate | Cosmetics Info". cosmeticsinfo.org. Archived from the original on 2017-09-29. Retrieved 2021-02-22.
