
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4,7,10,13,16-Hexaoxacyclooctadecane | |
| Identifiers | |
3D model (JSmol)
|
|
| 1619616 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.687 |
| EC Number |
|
| 4535 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H24O6 | |
| Molar mass | 264.315 g/mol |
| Density | 1.237 g/cm3 |
| Melting point | 37 to 40 °C (99 to 104 °F; 310 to 313 K) |
| Boiling point | 116 °C (241 °F; 389 K) (0.2 Torr) |
| 75 g/L | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Related compounds
|
Dibenzo-18-crown-6 Triglyme Hexaaza-18-crown-6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
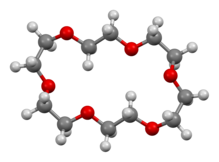
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point.[1] Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 is solvent- and temperature-dependent. Below 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene.[2] The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.
YouTube Encyclopedic
-
1/3Views:38 3594 565518 481
-
Properties of ethers and crown ethers | Organic chemistry | Khan Academy
-
Purple Benzene- exploring crown ethers
-
Posterior Crown Prep
Transcription
If we look at the boiling points of ethanol and dimethyl ether, we can see there's a large difference between them. Ethanol has a much higher boiling, 78 degrees Celsius. Whereas dimethyl ether is negative 25 degrees. And this explains the state of matter of these molecules. Ethanol, since its boiling point is higher than room temperature, is, of course, a liquid at room temperature and pressure. Whereas dimethyl ether, with a much lower boiling point, has already turned into a gas. And so we can explain the states of matter by looking at the intermolecular forces that are present in these molecules. So if I think about one molecule of ethanol, I know that the bonds between oxygen and hydrogen is polarized. I know that oxygen is more electronegative. So it will be partially negative. And the hydrogen is partially positive as it loses some electron density. If that molecule of ethanol interacts with another molecule of ethanol, the second molecule of ethanol is also polarized. The oxygen is partially negative. And the hydrogen is partially positive. And we know that opposite charges attract. So the partially positively-charged hydrogen is attracted to the partially negatively-charged oxygen like that. And there's going to be attraction between those two molecules. And we call this intermolecular force hydrogen bonding, the strongest type of intermolecular force. So hydrogen bonding is present between molecules of ethanol. And this accounts for its large boiling point. Let's look at more details about hydrogen bonding here. So hydrogen bonding exists when you have hydrogen bonded to an electronegative atom like that to this oxygen. But students forget that you also need another electronegative atom over here to give you more of a difference in charge and to make that hydrogen more partially positive. So it's really three atoms involved in hydrogen bonding there. Let's look at dimethyl ether and see why it does not exhibit hydrogen bonding. So if I were to draw one molecule of dimethyl ether here. And think about the polarization between the oxygen and this carbon right here. Oxygen is more electronegative. So it will be partially negative. This carbon will be partially positive like that. If I think about the interaction of that molecule of dimethyl ether with another molecule of dimethyl ether like that, you might be tempted to say, well there could be some hydrogen bonding because I know that this carbon right here has some hydrogens attached to it. And so some students will say, oh there must be hydrogen bonding between this oxygen down here and this hydrogen. But that is not the case because this hydrogen right here, while it is interacting with an oxygen, this hydrogen is bonded to a carbon which is not very electronegative. And so there's no large differences in electronegativity in the bond between carbon and hydrogen. Even the carbon's a little bit more electronegative. There's not enough to make this a true hydrogen bond. And so really there's only a small amount of dipole-dipole interaction between two molecules of dimethyl ether. So somewhere on this second molecule, there is a partial negative, partial positive. And so there will be a little bit of dipole-dipole interaction. But it's not very strong. And certainly nowhere near as strong as the hydrogen bonding exhibited on the left. Hydrogen bonding being just the super strong form of dipole-dipole interaction. And so dimethyl ether does not have as high of a boiling point as ethanol. Again, the answer is hydrogen bonding. Let's see what happens to the boiling point of ethers as we increase the number of carbons in the alkyl groups. So if we're going to look at that dimethyl ether again, and let's compare that to an ether that has more carbons than the alkyl group, so diethyl ether. We've already seen the boiling point of dimethyl ether as approximately negative 25 degrees Celsius. Whereas diethyl ether is about 35 degrees Celsius. And so there's a large difference in boiling points diethyl ethers boiling point is just higher than room temperature. So it is still a liquid at room temperature and pressure. So let's see if we can look at why diethyl ether has a higher boiling point. We know that ether molecules can't hydrogen bond with each other. So that cannot be the intermolecular force responsible for this increase in boiling point. So if we look at two molecules of diethyl ether interacting, one of the other intermolecular forces that we discussed was London dispersion forces. So London dispersion forces, you watched earlier video for more details. But when you have these large alkyl groups, provides more surface area for a form of attraction called London dispersion. And so that increased attraction between alkyl groups means that it's harder to pull those molecules apart. It requires more energy to pull those molecules apart, requires more heat in order to do so. And so that's the reason for the increase in boiling point that we see for diethyl ether, up to 35 degrees Celsius. And even though London dispersion forces are the weakest intermolecular forces, they're additive. So the effect is added when you have lots and lots of molecules. And that's the reason for the large difference between dimethyl ether and for diethyl ether. And so the increase of the number of carbons in the alkyl groups increases the boiling point just above room temperature but not much above room temperature. So this makes diethyl ether an excellent solvent for extraction. The other thing the alkyl groups do, is they increase the nonpolar part of the molecule. So it's a little bit more nonpolar due to these alkyl groups right here which means that diethyl ether is very good for dissolving a lot of nonpolar organic compounds. And so if you can dissolve a lot of nonpolar organic compounds and the boiling point is just above room temperature, it's an excellent solvent for extraction because you can dissolved are nonpolar organic molecules. And then you can just boil off the ether. And you're left with your organic product. So you'll use diethyl ether a lot for extractions. Let's look at another type of ether which is a kind of an interesting one. And we call these ethers, crown ethers. So if we look at that gigantic either there, it's called a crown ether. This was discovered by a guy named Charles Peterson who won the Nobel Prize for this. And the system of nomenclature for crown ethers would be to first count up how many atoms comprise your a ring here, your crown. So if we go 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, and 18. So there are 18 parts of this crown. So we would write an 18 right here like that, followed by the name crown, followed by the number of oxygens in here. So we have 1 oxygen, 2, 3, 4, 5, and 6. So the nomenclature would be, 18-crown-6 ether. And that just tells you what sort of crown ether that you are dealing with. So why is it called a crown ether? Well, the interesting thing about crown ethers are that they can interact with different ions. For example, the size of the potassium ion, so K+, happens to fit right in the center of this. So the spacing is just right for a potassium ion to fit in there. And since all of these oxygens have lone pairs of electrons on them, so negatively charged, there's an attraction between the positively-charged potassium ion and the negatively-charged electrons or the partially negative charge oxygen atoms. So there's attraction. Opposite charges attract. And those negative charges are going to hold that potassium ion in here like that. So it looks like a crown. If you think about the potassium ion as being someone's head and then that's wearing this ether crown on someone's head like that. And crown ethers have proved to be very useful, very useful things. For example, if you had some potassium fluoride, so some K+ F-. Well, normally potassium fluoride would not dissolve in a nonpolar organic solvents. But if you use a crown ether, the oxygens can take care of the potassium. And the outside of the crown either is nonpolar. So this portion and this portion, the outside of the crown ether is nonpolar which will dissolve in an organic solvent, in a nonpolar organic solvent like benzene like that. So like dissolves like. So this portion would dissolve in benzene. And then what that would do is, that would free up your fluoride anion. That would increase the nucleophilic strength of your fluoride anion which could participate in an SN2 reaction. So that's one of the uses of crown ethers is to go ahead and take the cation, leaving the anion to function as a better nucleophile because the potassium ion is solvated by the crown ether. And of course, since different kinds of different sizes, you can get different sized crown ethers to take care of those ions. So crown ethers I just think are very interesting molecules. And if you could look at a three dimensional representation of a crown ether, it's much easier to see that the outside is very nonpolar. So interesting, interesting molecules.
Synthesis
This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation:[3]
(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O
It can be also prepared by the oligomerization of ethylene oxide.[1] It can be purified by distillation, where its tendency to supercool becomes evident. 18-Crown-6 can also be purified by recrystallisation from hot acetonitrile. It initially forms an insoluble solvate.[3] Rigorously dry material can be made by dissolving the compound in THF followed by the addition of NaK to give [K(18-crown-6)]Na, an alkalide salt.[4]
Crystallographic analysis reveals a relatively flat molecule but one where the oxygen centres are not oriented in the idealized 6-fold symmetric geometry usually shown.[5] The molecule undergoes significant conformational change upon complexation.
Reactions

18-Crown-6 has a high affinity for the hydronium ion H3O+, as it can fit inside the crown ether. Thus, reaction of 18-crown-6 with strong acids gives the cation . For example, interaction of 18-crown-6 with HCl gas in toluene with a little moisture gives an ionic liquid layer with the composition , from which the solid can be isolated on standing. Reaction of the ionic liquid layer with two molar equivalents of water gives the crystalline product .[1][6][7]
Applications

18-Crown-6 binds to a variety of small cations, using all six oxygens as donor atoms. Crown ethers can be used in the laboratory as phase transfer catalysts.[8] Salts which are normally insoluble in organic solvents are made soluble by crown ether.[9] For example, potassium permanganate dissolves in benzene in the presence of 18-crown-6, giving the so-called "purple benzene", which can be used to oxidize diverse organic compounds.[1]
Various substitution reactions are also accelerated in the presence of 18-crown-6, which suppresses ion-pairing.[10] The anions thereby become naked nucleophiles. For example, using 18-crown-6, potassium acetate is a more powerful nucleophile in organic solvents:[1]
- [K(18-crown-6)+]OAc− + C6H5CH2Cl → C6H5CH2OAc + [K(18-crown-6)+]Cl−
The first electride salt to be examined with X-ray crystallography, [Cs(18-crown-6)2]+·e−, was synthesized in 1983. This highly air- and moisture-sensitive solid has a sandwich molecular structure, where the electron is trapped within nearly spherical lattice cavities. However, the shortest electron-electron distance is too long (8.68 Å) to make this material a conductor of electricity.[1]
References
- ^ a b c d e f Steed, Jonathan W.; Atwood, Jerry L. (2009). Supramolecular Chemistry (2nd ed.). Wiley. ISBN 978-0-470-51233-3.
- ^ Caswell, Lyman R.; Savannunt, Diana S. (January 1988). "Temperature and solvent effects on the experimental dipole moments of three crown ethers". J. Heterocyclic Chem. 25 (1): 73–79. doi:10.1002/jhet.5570250111.
- ^ a b Gokel, George W.; Cram, Donald J.; Liotta, Charles L.; Harris, Henry P.; Cook, Fred L. (1977). "18-Crown-6". Org. Synth. 57: 30. doi:10.15227/orgsyn.057.0030.
- ^ Jilek, Robert E.; Fischer, Paul J.; Ellis, John E. (2014). "Bis(1,2-Bis(Dimethylphosphano)Ethane)Tricarbonyltitanium(0) and Hexacarbonyltitanate(2−)". Inorganic Syntheses: Volume 36. Vol. 36. pp. 127–134. doi:10.1002/9781118744994.ch24. ISBN 9781118744994.
- ^ Dunitz, J. D.; Seiler, P. (1974). "1,4,7,10,13,16-Hexaoxacyclooctadecane". Acta Crystallogr. B30 (11): 2739. doi:10.1107/S0567740874007928.
- ^ Atwood, Jerry L.; Bott, Simon G.; Coleman, Anthony W.; Robinson, Kerry D.; Whetstone, Stephen B.; Means, C. Mitchell (December 1987). "The oxonium cation in aromatic solvents. Synthesis, structure, and solution behavior of ". Journal of the American Chemical Society. 109 (26): 8100–8101. doi:10.1021/ja00260a033.
- ^ Atwood, Jerry L.; Bott, Simon G.; Means, C. Mitchell; Coleman, Anthony W.; Zhang, Hongming; May, Michael T. (February 1990). "Synthesis of salts of the hydrogen dichloride anion in aromatic solvents. 2. Syntheses and crystal structures of and the related ". Inorganic Chemistry. 29 (3): 467–470. doi:10.1021/ic00328a025.
- ^ Liotta, C. L.; Berknerin, J. (2004). "18-Crown-6". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rc261. ISBN 0471936235.
- ^ Wynn, David; et al. (1984). "The Solubility of Alkali-Metal Fluorides in Non-Aqueous Solvents With and Without Crown Ethers...". Talanta. 31 (11): 1036–1040. doi:10.1016/0039-9140(84)80244-1. PMID 18963717.
- ^ Cook, Fred L.; Bowers, Chauncey W.; Liotta, C. L. (November 1974). "Chemistry of naked anions. III. Reactions of the 18-crown-6 complex of potassium cyanide with organic substrates in aprotic organic solvents". The Journal of Organic Chemistry. 39 (23): 3416–3418. doi:10.1021/jo00937a026.

![{\displaystyle {\ce {[H3O.18-crown-6]+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/05959c612544dc9b3cb3553c23497b6cb0b79eae)
![{\displaystyle {\ce {[H3O.18-crown-6]+[HCl2]^{-}.}}3.8{\ce {C6H5Me}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6c99a07eb7055bb324034bb06c5b326acac2040)
![{\displaystyle {\ce {[H3O.18-crown-6]+[HCl2]-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a39f9fa425e04e4fb9893a6534ec9ebe14ec13d)
![{\displaystyle {\ce {(H5O2)[H3O.18-crown-6]Cl2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ea4d202cf03dca6460e22c323e76cdeaa4e4da33)
![{\displaystyle {\ce {[H3O+.18-crown-6][Cl-H-Cl]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/570b60b09f20cda7d97987382a006e776dd9ed9e)
![{\displaystyle {\ce {[K.18-crown-6][Cl-H-Cl]}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/033a571d20719af08d7a5198f57da59afc23a226)
![{\displaystyle {\ce {[Mg.18-crown-6][Cl-H-Cl]2}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/abf6c19ea8d8195eea1d4679f2a696d69feb2f72)
![{\displaystyle {\ce {[H3O.18-crown-6][Cl-H-Cl]}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/53c829dc937cd5208e3ccb616bdddf618c8b6332)
![{\displaystyle {\ce {[H3O.18-crown-6][Br-H-Br]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d1dbd94a5bd7bbec553563db4f4df5d9496b9cf1)