| |

| |
| Names | |
|---|---|
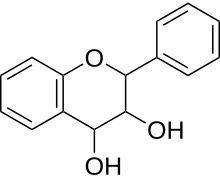
| IUPAC name
Flavan-3,4-diol
| |
| Systematic IUPAC name
2-Phenyl-3,4-dihydro-2H-1-benzopyran-3,4-diol | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C15H14O3 | |
| Molar mass | 242.274 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.[citation needed]
Such compounds include:
- Leucocyanidin
- Leucodelphinidin
- Leucofisetinidin
- Leucomalvidin
- Leucopelargonidin
- Leucopeonidin
- Leucorobinetinidin
- Melacacidin
- Teracacidin from Acacia obtusifolia and Acacia maidenii heartwoods[1]
Leucoanthocyanidins have been demonstrated to be intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana.[2]
Bate-smith recommended in 1954 the use of the Forestal solvent for the isolation of leuco-anthocyanins.[3]
YouTube Encyclopedic
-
1/3Views:1 0713 1531 570
-
ANTHOCYANINS - PART-3
-
FLAVONOIDS - PART 1: Chemistry of Flavonoids, Classification of Flavonoids
-
„Yopo & Vilca” Meditation | Isochronic Tones + Binaural Beats | Tribute To Sacred Shamanic Snuffs
Transcription
Metabolism
Leucoanthocyanidin dioxygenase uses flavan-3,4-diols to produce 3-hydroxyanthocyanidins.[4] The gene encoding the enzyme (PpLDOX) has been identified in peach[5] and expression has been studied in Vitis vinifera.[6]
References
- ^ Clark-Lewis, JW; Dainis, I (1967). "Flavan derivatives. XIX. Teracacidin and isoteracacidin from Acacia obtusifolia and Acacia maidenii heartwoods; Phenolic hydroxylation patterns of heartwood flavonoids characteristic of sections and subsections of the genus Acacia". Australian Journal of Chemistry. 20 (10): 2191–2198. doi:10.1071/CH9672191.
- ^ Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta. 163 (2): 191–196. doi:10.1007/BF00393505. PMID 24249337. S2CID 20854538.
- ^ Bate-SMITH, EC (September 1954). "Leuco-anthocyanins. 1. Detection and identification of anthocyanidins formed leuco-anthocyanins in plant tissues". Biochem. J. 58 (1): 122–5. doi:10.1042/bj0580122. PMC 1269852. PMID 13198862.
- ^ "leucoanthocyanidin dioxygenase on mondofacto.com". Archived from the original on 2012-03-04. Retrieved 2009-09-03.
- ^ Ogundiwin, E. A.; Peace, C. P.; Nicolet, C. M.; Rashbrook, V. K.; Gradziel, T. M.; Bliss, F. A.; Parfitt, D.; Crisosto, C. H. (2008). "Leucoanthocyanidin dioxygenase gene (PpLDOX): A potential functional marker for cold storage browning in peach". Tree Genetics & Genomes. 4 (3): 543–554. doi:10.1007/s11295-007-0130-0. S2CID 22579882.
- ^ Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera, Gollop R; Farhi S.; Perl A. 2001
