| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
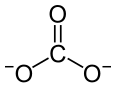
| Eu2(CO3)3 | |
| Molar mass | 483.961 g/mol |
| Appearance | Solid |
| Melting point | Decomposes |
| Insoluble (1.94×10-6mol/L,30℃)[1] | |
| Related compounds | |
Other cations
|
Samarium(III) carbonate Gadolinium(III) carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Europium(III) carbonate is an inorganic compound with the chemical formula Eu2(CO3)3.
YouTube Encyclopedic
-
1/2Views:862588
-
Europium Explained in 20 Minutes or Less
-
Europium - Tales from the Periodic Table
Transcription
Preparation
Europium(III) carbonate can be obtained by mixing and heating an aqueous solution of ammonium carbonate and europium(III) chloride.[2] A saturated carbon dioxide ammonium carbonate solution (obtained from the reaction of hydrochloric acid and ammonium carbonate solution) can also precipitate europium carbonate from a europium salt solution.[3] Other preparation methods include the thermal decomposition of europium(III) acetate[4] and the reaction of a suspension of europium(III) oxide in water and supercritical carbon dioxide.[5]
Chemical properties
Europium(III) carbonate is soluble in acid and releases carbon dioxide:[1]
- Eu2(CO3)3 + 6 H+ → 2 Eu+3 + 3 H2O + 3 CO2↑
Europium(III) carbonate decomposes at high temperatures to form europium(III) oxide:
- Eu2(CO3)3 → Eu2O3 + 3 CO2↑
References
- ^ a b 《无机化学丛书》. 第七卷 钪 稀土元素. 易宪武 黄春晖 等编.科学出版社. P174. Carbonates. ISBN 978-7-03-030574-9
- ^ R.G. Charles (Jul 1965). "Rare-earth carbonates prepared by homogeneous precipitation". Journal of Inorganic and Nuclear Chemistry. 27 (7): 1489–1493. doi:10.1016/0022-1902(65)80008-2. Archived from the original on 2018-06-12. Retrieved 2020-04-23.
- ^ Perkovskaya, Yu. B.; Anoshina, N. P.; Sukhanova, I. M. Rare earth carbonates. Metody Polucheniya Khimicheskikh Reaktivov i Preparatov, 1967. 16: 104-109. ISSN: 0539-5143.
- ^ E.L. Head (Feb 1966). "Preparation of the carbonates of the rare earths from some of their organic acid salts". Inorganic and Nuclear Chemistry Letters. 2 (2): 33–37. doi:10.1016/0020-1650(66)80087-9. Archived from the original on 2018-06-18. Retrieved 2020-04-23.
- ^ Yanagihara, N.; Vemulapalli, K.; Fernando, Q. Synthesis of lanthanide carbonates using supercritical carbon dioxide. Kidorui, 1991. 18: 136-137. ISSN: 0910-2205.
