A bioreactor refers to any manufactured device or system that supports a biologically active environment.[1] In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel.[citation needed] It may also refer to a device or system designed to grow cells or tissues in the context of cell culture.[2] These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.[citation needed]
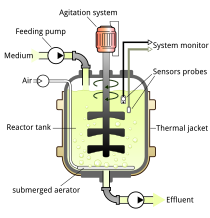
On the basis of mode of operation, a bioreactor may be classified as batch, fed batch or continuous (e.g. a continuous stirred-tank reactor model). An example of a continuous bioreactor is the chemostat.[citation needed]
Organisms or biochemically active substances growing in bioreactors may be submerged in liquid medium or may be anchored to the surface of a solid medium. Submerged cultures may be suspended or immobilized. Suspension bioreactors may support a wider variety of organisms, since special attachment surfaces are not needed, and can operate at a much larger scale than immobilized cultures. However, in a continuously operated process the organisms will be removed from the reactor with the effluent. Immobilization is a general term describing a wide variety of methods for cell or particle attachment or entrapment.[3] It can be applied to basically all types of biocatalysis including enzymes, cellular organelles, animal and plant cells and organs.[4][5] Immobilization is useful for continuously operated processes, since the organisms will not be removed with the reactor effluent, but is limited in scale because the microbes are only present on the surfaces of the vessel.
Large scale immobilized cell bioreactors are:
- moving media, also known as moving bed biofilm reactor (MBBR)
- packed bed
- fibrous bed
- membrane
YouTube Encyclopedic
-
1/5Views:540 41663 5559 020142 14156 734
-
Bioprocessing Part 1: Fermentation
-
Reactor Sampling Process Animation
-
Part 4: Cell production at benchtop scale, harvest and purification
-
Chemical Reactor Animation
-
Imperfect Mixing in a Stirred Tank Reactor Demonstration
Transcription
>>We all know something about fermentation. It's a process used countless times each day to make a variety of dairy products, baked goods and beverages. We sometimes think of it as letting foods go bad, but in a controlled way. With a little help, milk becomes yogurt... bread rises... and grains decompose, creating alcoholic beverages and alternative fuels. >>But looking at these examples only gives us a clue as to what's really happening and how we can use the power of fermentation to cost-effectively create a broad array of biological products. >>So, what is fermentation? A cell can be thought of as a micro-factory. These cells can be bacteria, fungi or specific cells from mammals, plants or insects. In Biotechnology, these cells are used to manufacture a product in a process called fermentation. >>For yogurt, buttermilk and cheese, we use bacteria. To make breads and alcoholic beverages we use yeast - a fungus! And the production of some vaccines requires the growth of mammalian cells that are infected with a specific virus. >>The product the cells manufacture is usually a chemical the cells contain naturally... or a substance that the cells have been genetically altered to create... or even a metabolic waste product of the organism's growth - like one of our examples, alcohol! >>There are too many everyday products created by commercial-scale fermentation to even list, but some common ones include: amino acids, biopharmaceuticals, dyes, enzymes, food products, lipids, steroids and vitamins. [Music Fades, Nat Sound of Process Establishes, Under For VO] >>Fermentation is a reasonably simple process. A cell is selected based on its ability to produce the desired product. A seed stock of cells is put into a small amount of media. Media provides the nutritional products the cell needs to grow. When the population of cells has grown and consumed most of the nutrients, it's moved into a larger vessel with more growth media, and the process repeats... This "scaling-up" is complete when the quantity of cells is large and healthy enough to transfer into a production vessel - often referred to as a bioreactor or fermentor. >>With plenty of fresh media now available and under tightly controlled conditions, the cells grow and manufacture product. When the fermentation is complete, the product is harvested. >>Fermentation is known as an "upstream" biotechnology process. It occurs early in the production flow, before Recovery, Purification, Formulation, Filling and Packaging. To better understand the fermentation process, we should first find out a little bit about the cells we use and what they may require to reproduce and stay healthy. Different cells have different needs. Some are aerobic - they need oxygen - while others are anaerobic and do not require oxygen. >>All cells require nutrition. A properly formulated media contains the necessary nutrients to allow cells to grow and produce. The fermentor mixes the cells evenly throughout the media to suspend the cells and supply the oxygen necessary for growth. Effective and efficient fermentation requires rigorous monitoring and control of the environment within the bioreactor. Key factors include temperature, pressure, pH - which is a measure of how acidic or alkaline the media is, oxygen - usually measured as dissolved oxygen within the media, and nutrient levels. Although the environment and the media are tailored to the needs of specific cells, the lifecycle of almost all batches follows a predictable pattern. The growth pattern has four phases: Lag, Exponential or Log, Stationary and Death. >>When a cell is first introduced to fresh media, it has to adapt to its new environment. This creates a lull or Lag in the growth timeline. After the organism adapts, the batch takes off! The cells begin dividing at a constant rate - an Exponential or Logarithmic (or "Log") increase; doubling, then doubling again, and on and on... As the nutrients in the media are consumed, toxic metabolic waste products build up, cells begin to die, and growth slows. When it reaches the point that just as many cells are dying as are dividing, the batch enters the Stationary phase. This is the point at which the key nutrients are completely consumed, the fermentation is stopped and the fermented broth is harvested. If the fermentation were allowed to continue, the cells would enter the Death phase. More cells die than divide, and - similar to the Exponential phase - the death rate increases logarithmically. Now that we have a basic understanding of how Fermentation works, let's look at an actual process and see how it all comes together. For our sample process we will look at the production of Green Fluorescent Protein, or GFP. GFP is broadly used as a biological marker. It's a fluorescent dye that's very well tolerated by most cells and doesn't interfere with normal cellular function.. In the GFP fermentation process, we'll need to add an antibiotic to protect the purity of the batch, and then - late in the process - a biochemical inducer to "turn on" the GFP gene Our materials for this process will include: A bacteria seed stock - in this case E. coli - that has been genetically enhanced to produce GFP... the basic ingredients for a compatible media which include nutrients, stabilizers, an antibiotic and an antifoaming agent... and IPTG which is the biochemical inducer that "switches-on" the GFP gene. The equipment that we'll be using includes a 300 liter bioreactor, a UV/Vis Spectrophotometer to monitor the optical density, which is a measure of the concentration of cells in the bioreactor - a glucose analyzer, to measure glucose, a key nutrient - an off-line, pH meter to help track the acid/base balance, and adjust on-line measurements, if needed... and a Broth Tank for our final product. The bioreactor is equipped with a water jacket around the vessel to regulate temperature, and integrated sensors to monitor key environmental factors, including dissolved oxygen, pH, internal temperature, water-jacket temperature and vessel pressure. The reactor also has an agitator, dedicated ports for adding seed stock and media ingredients, separate ports for acid and base supplement, air filters for supply and exhaust, and valves for drawing samples and for harvesting. Most fermentation and monitoring functions can be managed from the bioreactor's dedicated process controller. Before the fermentation process can begin, the area must be prepared. Preparation includes removing equipment and material that won't be used in the process... Cleaning and sanitizing the area and equipment... and sterilizing equipment as required by the SOPs - Standard Operating Procedures. Sterilization is used to eliminate unwanted microorganisms which can grow naturally in the fermentation media and process equipment. Also, all required materials and documentation should be gathered and prepared... and all Process Control software should be loaded and verified. The Fermentation batch process will be guided and documented with the BPR - Batch Process Record. The Batch Record leads the operator through the process, step-by-step... with each step requiring a sign-off and separate verification. This record also includes spaces for documenting key times, activities and instrument readings. The GFP fermentation process really begins with the expansion of our bacteria seed stock. After removing the specially modified E-coli from the freezer and thawing it... It's used to inoculate a small amount of fresh media in a shaker flask. After the number of cells has reached the target amount, the thriving cells are ready for fermentation. Meanwhile, in the Fermentation area, operators begin with a complete check of all critical equipment. Valves, caps and lines are checked, hoses are tightened... probes are verified and calibrated. and 10 kilograms of HPW - High Purity Water - is added to the vessel. The bioreactor is brought up to normal process pressure and held there in order to check for leaks. The pressure is monitored over a 30 minute period. If a leak is detected, the problem is corrected and the test is run again. Once the reactor passes the test, we are ready to mix the media in the vessel. The agitator is turned on, and the ingredients are added: Yeast Extract... Tryptic Soy Broth... Ammonium chloride... Sodium biphosphate... Monopotassium phosphate... and an Antifoam compound. Once all the initial ingredients are in, another 10 kilograms of High Purity Water is added... all ports and valves are closed... all condensate valves are opened... and the bioreactor begins an SIP - Sterilize-In-Place cycle. The target for sterilization is 121 degrees Celsius for 30 minutes. As soon as the temperature climbs to the targeted temperature, the condensate valves are closed, and the SIP cycle completes automatically. Both the vessel and the media are now sterile - And we're ready to add the final ingredients to our media. The glucose hose is attached to the vessel the connection is steamed to sterilize it and the separately sterilized glucose-antibiotic solution is pumped into the vessel. Then a manual pH reading of the media is taken and the bioreactor is set up for its fermentation cycle. After the inoculation hose is connected to the reactor and steamed for 20 minutes the expanded seed stock is pumped into the reactor containing the media. Fermentation now begins. The operator takes zero hour readings and begins to regularly monitor batch temperature, agitator RPMs, dissolved oxygen levels, pH, vessel pressure, optical density, air flow rate and glucose concentrations. Optical Densities and glucose concentrations are of particular interest, so they're graphed as well as documented. When the targeted levels of glucose and optical density are achieved, it's time to add IPTG to the vessel to activate or turn on the expression of the Green Fluorescent Protein in the cells. After allowing enough time for the cells to produce green fluorescent protein usually 5 hours more, final readings are taken and a sample is drawn to check the percentage of cell solids. The product is now referred to as "broth." The broth, which contains spent media and cells, is complete when the key nutrient, glucose, is mostly consumed, and the batch has reached the desired concentration. The batch is then cooled down, pumped into a broth tank... and labeled with the batch number, volume, time and date. [Bright, Rhythm-Driven Music Establishes, Then Under For VO] The Fermentation process is now complete! The harvested broth will now move downstream to the Recovery process where the cells will be ruptured to free the Green Fluorescent Protein and the protein will be separated from the other broth components.
Design


Bioreactor design is a relatively complex engineering task, which is studied in the discipline of biochemical/bioprocess engineering. Under optimum conditions, the microorganisms or cells are able to perform their desired function with limited production of impurities. The environmental conditions inside the bioreactor, such as temperature, nutrient concentrations, pH, and dissolved gases (especially oxygen for aerobic fermentations) affect the growth and productivity of the organisms. The temperature of the fermentation medium is maintained by a cooling jacket, coils, or both. Particularly exothermic fermentations may require the use of external heat exchangers. Nutrients may be continuously added to the fermenter, as in a fed-batch system, or may be charged into the reactor at the beginning of fermentation. The pH of the medium is measured and adjusted with small amounts of acid or base, depending upon the fermentation. For aerobic (and some anaerobic) fermentations, reactant gases (especially oxygen) must be added to the fermentation. Since oxygen is relatively insoluble in water (the basis of nearly all fermentation media), air (or purified oxygen) must be added continuously. The action of the rising bubbles helps mix the fermentation medium and also "strips" out waste gases, such as carbon dioxide. In practice, bioreactors are often pressurized; this increases the solubility of oxygen in water. In an aerobic process, optimal oxygen transfer is sometimes the rate limiting step. Oxygen is poorly soluble in water—even less in warm fermentation broths—and is relatively scarce in air (20.95%). Oxygen transfer is usually helped by agitation, which is also needed to mix nutrients and to keep the fermentation homogeneous. Gas dispersing agitators are used to break up air bubbles and circulate them throughout the vessel.[citation needed]
Fouling can harm the overall efficiency of the bioreactor, especially the heat exchangers. To avoid it, the bioreactor must be easily cleaned. Interior surfaces are typically made of stainless steel for easy cleaning and sanitation. Typically bioreactors are cleaned between batches, or are designed to reduce fouling as much as possible when operated continuously. Heat transfer is an important part of bioreactor design; small vessels can be cooled with a cooling jacket, but larger vessels may require coils or an external heat exchanger.[citation needed]
Types
Photobioreactor

A photobioreactor (PBR) is a bioreactor which incorporates some type of light source (that may be natural sunlight or artificial illumination). Virtually any translucent container could be called a PBR, however the term is more commonly used to define a closed system, as opposed to an open storage tank or pond. Photobioreactors are used to grow small phototrophic organisms such as cyanobacteria, algae, or moss plants.[6] These organisms use light through photosynthesis as their energy source and do not require sugars or lipids as energy source. Consequently, risk of contamination with other organisms like bacteria or fungi is lower in photobioreactors when compared to bioreactors for heterotroph organisms.[citation needed]
Sewage treatment
Conventional sewage treatment utilises bioreactors to undertake the main purification processes. In some of these systems, a chemically inert medium with very high surface area is provided as a substrate for the growth of biological film. Separation of excess biological film takes place in settling tanks or cyclones. In other systems aerators supply oxygen to the sewage and biota to create activated sludge in which the biological component is freely mixed in the liquor in "flocs". In these processes, the liquid's biochemical oxygen demand (BOD) is reduced sufficiently to render the contaminated water fit for reuse. The biosolids can be collected for further processing, or dried and used as fertilizer. An extremely simple version of a sewage bioreactor is a septic tank whereby the sewage is left in situ, with or without additional media to house bacteria. In this instance, the biosludge itself is the primary host for the bacteria.[citation needed]
Bioreactors for specialized tissues

Many cells and tissues, especially mammalian ones, must have a surface or other structural support in order to grow, and agitated environments are often destructive to these cell types and tissues. Higher organisms, being auxotrophic, also require highly specialized growth media. This poses a challenge when the goal is to culture larger quantities of cells for therapeutic production purposes, and a significantly different design is needed compared to industrial bioreactors used for growing protein expression systems such as yeast and bacteria.[citation needed]
Many research groups have developed novel bioreactors for growing specialized tissues and cells on a structural scaffold, in attempt to recreate organ-like tissue structures in-vitro. Among these include tissue bioreactors that can grow heart tissue,[7][8] skeletal muscle tissue,[9] ligaments, cancer tissue models, and others. Currently, scaling production of these specialized bioreactors for industrial use remains challenging and is an active area of research.
For more information on artificial tissue culture, see tissue engineering.
Modelling
Mathematical models act as an important tool in various bio-reactor applications including wastewater treatment. These models are useful for planning efficient process control strategies and predicting the future plant performance. Moreover, these models are beneficial in education and research areas.[citation needed]
Bioreactors are generally used in those industries which are concerned with food, beverages and pharmaceuticals. The emergence of biochemical engineering is of recent origin. Processing of biological materials using biological agents such as cells, enzymes or antibodies are the major pillars of biochemical engineering. Applications of biochemical engineering cover major fields of civilization such as agriculture, food and healthcare, resource recovery and fine chemicals.[citation needed]
Until now, the industries associated with biotechnology have lagged behind other industries in implementing control over the process and optimization strategies. A main drawback in biotechnological process control is the problem of measuring key physical and biochemical parameters.[10]
Operational stages in a bio-process
A bioprocess is composed mainly of three stages—upstream processing, bioreaction, and downstream processing—to convert raw material to finished product.[11]
The raw material can be of biological or non-biological origin. It is first converted to a more suitable form for processing. This is done in an upstream processing step which involves chemical hydrolysis, preparation of liquid medium, separation of particulate, air purification and many other preparatory operations.[citation needed]
After the upstream processing step, the resulting feed is transferred to one or more bioreaction stages. The biochemical reactors or bioreactors form the base of the bioreaction step. This step mainly consists of three operations, namely, production of biomass, metabolite biosynthesis and biotransformation.[citation needed]
Finally, the material produced in the bioreactor must be further processed in the downstream section to convert it into a more useful form. The downstream process mainly consists of physical separation operations which include solid liquid separation, adsorption, liquid-liquid extraction, distillation, drying etc.[12]
Specifications
A typical bioreactor consists of following parts:
Agitator – Used for the mixing of the contents of the reactor which keeps the cells in the perfect homogenous condition for better transport of nutrients and oxygen to the desired product(s).
Baffle – Used to break the vortex formation in the vessel, which is usually highly undesirable as it changes the center of gravity of the system and consumes additional power.
Sparger – In aerobic cultivation process, the purpose of the sparger is to supply adequate oxygen to the growing cells.
Jacket – The jacket provides the annular area for circulation of constant temperature of water which keeps the temperature of the bioreactor at a constant value.[13]
See also
- ATP test
- Biochemical engineering
- Biofuel from algae
- Biological hydrogen production (algae)
- Bioprocessor
- Bioreactor landfill
- Biotechnology
- Cell culture
- Chemostat
- Digester
- Electro-biochemical reactor (EBR)
- Hairy root culture
- History of biotechnology
- Hollow fiber bioreactor
- Immobilized enzyme
- Industrial biotechnology
- Moving bed biofilm reactor
- Septic tank
- Single-use bioreactor
- Tissue engineering
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bioreactor". doi:10.1351/goldbook.B00662
- ^ "Bioreactoes and Cultivation Systems for Cell and Tissue Culture" (PDF). eolss.net. Retrieved 12 August 2023.
- ^ López, Asunción; Lázaro, Nuria; Marqués, Ana M. (September 1997). "The interphase technique: a simple method of cell immobilization in gel-beads". Journal of Microbiological Methods. 30 (3): 231–234. doi:10.1016/S0167-7012(97)00071-7.
- ^ Kowalczyk, Tomasz; Sitarek, Przemysław; Toma, Monika; Rijo, Patricia; Domínguez‐Martín, Eva; Falcó, Irene; Sánchez, Gloria; Śliwiński, Tomasz (August 2021). "Enhanced Accumulation of Betulinic Acid in Transgenic Hairy Roots of Senna obtusifolia Growing in the Sprinkle Bioreactor and Evaluation of Their Biological Properties in Various Biological Models". Chemistry & Biodiversity. 18 (8): e2100455. doi:10.1002/cbdv.202100455. hdl:10261/247635. ISSN 1612-1872. PMID 34185351. S2CID 235672736.
- ^ Peinado, Rafael A.; Moreno, Juan J.; Villalba, Jose M.; González-Reyes, Jose A.; Ortega, Jose M.; Mauricio, Juan C. (December 2006). "Yeast biocapsules: A new immobilization method and their applications". Enzyme and Microbial Technology. 40 (1): 79–84. doi:10.1016/j.enzmictec.2005.10.040.
- ^ Decker, Eva L.; Reski, Ralf (14 August 2007). "Current achievements in the production of complex biopharmaceuticals with moss bioreactors". Bioprocess and Biosystems Engineering. 31 (1): 3–9. doi:10.1007/s00449-007-0151-y. PMID 17701058. S2CID 4673669.
- ^ Bursac, N.; Papadaki, M.; Cohen, R. J.; Schoen, F. J.; Eisenberg, S. R.; Carrier, R.; Vunjak-Novakovic, G.; Freed, L. E. (1 August 1999). "Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies". American Journal of Physiology. Heart and Circulatory Physiology. 277 (2): H433–H444. doi:10.1152/ajpheart.1999.277.2.h433. PMID 10444466.
- ^ Carrier, Rebecca L.; Papadaki, Maria; Rupnick, Maria; Schoen, Frederick J.; Bursac, Nenad; Langer, Robert; Freed, Lisa E.; Vunjak-Novakovic, Gordana (5 September 1999). "Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization". Biotechnology and Bioengineering. 64 (5): 580–589. doi:10.1002/(SICI)1097-0290(19990905)64:5<580::AID-BIT8>3.0.CO;2-X. PMID 10404238.
- ^ Heher, Philipp; Maleiner, Babette; Prüller, Johanna; Teuschl, Andreas Herbert; Kollmitzer, Josef; Monforte, Xavier; Wolbank, Susanne; Redl, Heinz; Rünzler, Dominik; Fuchs, Christiane (September 2015). "A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain". Acta Biomaterialia. 24: 251–265. doi:10.1016/j.actbio.2015.06.033. PMID 26141153.
- ^ Carlsson, Bengt (March 24, 2009). "An introduction to modeling of bioreactors" (PDF).
- ^ Rosser, J.; Thomas, D. J. (2018-01-01), Thomas, Daniel J.; Jessop, Zita M.; Whitaker, Iain S. (eds.), "10 - Bioreactor processes for maturation of 3D bioprinted tissue", 3D Bioprinting for Reconstructive Surgery, Woodhead Publishing, pp. 191–215, ISBN 978-0-08-101103-4, retrieved 2020-12-14
- ^ Jana, AMIYA K. (2011). CHEMICAL PROCESS MODELLING AND COMPUTER SIMULATION. PHI Learning Pvt. Ltd.[page needed]
- ^ "Bioreactor- Basics".
Further reading
- Pauline M Doran, Bio-process Engineering Principles, Elsevier, 2nd ed., 2013 ISBN 978-0-12-220851-5
- Biotechnology company

