| |
| Names | |
|---|---|
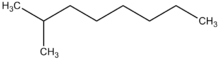
| Preferred IUPAC name
2-Methyloctane[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 1696917 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.771 |
| EC Number |
|
| 240576 | |
| MeSH | nonane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1920 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H20 | |
| Molar mass | 128.259 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 0.718 g/mL |
| Melting point | −54.1 to −53.1 °C; −65.5 to −63.7 °F; 219.0 to 220.0 K |
| Boiling point | 150.4 to 151.0 °C; 302.6 to 303.7 °F; 423.5 to 424.1 K |
| log P | 5.293 |
| Vapor pressure | 0.59 kPa (at 25.0 °C) |
Henry's law
constant (kH) |
1.7 nmol Pa−1 kg−1 |
| −108.13×10−6 cm3/mol | |
Refractive index (nD)
|
1.405 |
| Thermochemistry | |
Heat capacity (C)
|
284.34 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
393.67 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−275.7 – −273.7 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−6125.75 – −6124.67 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H226, H304, H315, H319, H332, H336 | |
| P261, P301+P310, P305+P351+P338, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 31.0 °C (87.8 °F; 304.1 K) |
| 205.0 °C (401.0 °F; 478.1 K) | |
| Explosive limits | 0.87–2.9% |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[2] |
REL (Recommended)
|
TWA 200 ppm (1050 mg/m3)[2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methyloctane is a branched alkane hydrocarbon with the chemical formula C9H20. It is a colorless, flammable liquid
Combustion reactions
2-Methyloctane burns in the same way as other alkanes. Where there is enough oxygen, nonane burns to form water and carbon dioxide, so 2-methyloctane would do the same.
When insufficient oxygen is present for complete combustion, carbon monoxide is produced.
- 2 C9H20 + 19 O2 → 18 CO + 20 H2O
See also
References
- ^ "nonane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 6 January 2012.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0466". National Institute for Occupational Safety and Health (NIOSH).
- ^ "NFPA Hazard Rating Information for Common Chemicals". Archived from the original on 2015-02-17. Retrieved 2015-03-13.

