 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral; injection (intramuscular or slow intravenous); topical (ophthalmic/nasal solution) |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic hydroxylation, demethylation and glucuronidation |
| Elimination half-life | 16 - 19 hrs (oral), 8 - 7 hrs (i.v.)[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.506 |
| Chemical and physical data | |
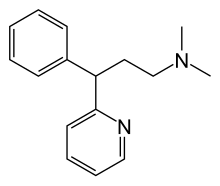
| Formula | C16H20N2 |
| Molar mass | 240.350 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pheniramine (trade name Avil among others) is an antihistamine with anticholinergic properties used to treat allergic conditions such as hay fever or urticaria. It has relatively strong sedative effects, and may sometimes be used off-label as an over-the-counter sleeping pill in a similar manner to other sedating antihistamines such as diphenhydramine. Pheniramine is also commonly found in eyedrops used for the treatment of allergic conjunctivitis.
It was patented in 1948.[2] Pheniramine is generally sold in combination with other medications, rather than as a stand-alone drug, although some formulations are available containing pheniramine by itself.
YouTube Encyclopedic
-
1/5Views:19 3551 59245 68913 4952 296
-
Avil 25 tablet use full review in hindi
-
Dexamethasone : Indications, Contraindications, Dose, Mechanism of action
-
H1 Blockers or Antihistamines - 1st Generation & 2nd Generation Drugs
-
Addict to Fortwin & Phenargan having multiple ulcers on both lower limbs - Dr Narotam Dewan
-
Classification tricks of H1 antagonists
Transcription
Side effects
Pheniramine may cause drowsiness or Tachycardia, and over-dosage may lead to sleep disorders.[citation needed]
Overdose may lead to seizures, especially in combination with alcohol.[citation needed]
People combining with cortisol in the long term should avoid pheniramine as it may decrease levels of adrenaline (epinephrine) which may lead to loss of consciousness.[citation needed]
Pheniramine is a deliriant (hallucinogen) in toxic doses. Recreational use of Coricidin for the dissociative (hallucinogenic) effect of its dextromethorphan is hazardous because it also contains chlorpheniramine.[citation needed]
Chemical relatives
Halogenation of pheniramine increases its potency 20-fold. Halogenated derivatives of pheniramine include chlorphenamine, brompheniramine, dexchlorpheniramine, dexbrompheniramine, and zimelidine. Two other halogenated derivatives, fluorpheniramine and iodopheniramine, are currently in use for research on combination therapies for malaria and some cancers. [citation needed]
Other analogs include diphenhydramine, and doxylamine.
Stereoisomerism
Pheniramine contains a stereocenter and can exists as either of two enantiomers. The pharmaceutical drug is a racemate, an equal mixture of the (R)- and (S)-forms.[3]
| Enantiomers of pheniramine | |
|---|---|
 (R)-Pheniramine CAS number: 56141-72-1 |
 (S)-Pheniramine CAS number: 23201-92-5 |
See also
References
- ^ Witte PU, Irmisch R, Hajdú P (January 1985). "Pharmacokinetics of pheniramine (Avil) and metabolites in healthy subjects after oral and intravenous administration". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 23 (1): 59–62. PMID 3988394.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 546. ISBN 9783527607495.
- ^ von Bruchhausen F, Dannhardt G, Ebel S, Frahm AW, Hackenthal E, Holzgrabe U (2014). Hagers Handbuch der Pharmazeutischen Praxis. Vol. Band 9: Stoffe P-Z (5th ed.). Berlin: Springer Verlag. p. 121. ISBN 978-3-642-63389-8.
External links
 Media related to Pheniramine at Wikimedia Commons
Media related to Pheniramine at Wikimedia Commons- MedlinePlus Encyclopedia: Pheniramine overdose
- Leaflet on Avil by The Royal Australian College of General Practitioners
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
