 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, nasal, IV |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
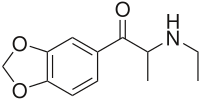
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone (MDEC, βk-MDEA), is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone.[citation needed] In the United States, it began to be found in cathinone products in late 2011.[3]
Very little data exists about the pharmacological properties, metabolism, and toxicity of ethylone, and although several ethylone-related deaths have been reported, the cause of death was not due to ingestion of ethylone.[3]
YouTube Encyclopedic
-
1/5Views:274 768617 9322 250181 689373 809
-
“Research Chemicals” - RC Safety Guide
-
How to extract chemicals from over the counter products
-
Reagent Tests Part 1: Mandelin, Marquis, and Mecke Reagents
-
MDA: What You Need To Know
-
Methamphetamine: What You Need To Know
Transcription
Pharmacokinetics
Analysis of human and rat urine for the metabolites of bk-amphetamines suggested that ethylone was degraded in the following metabolic steps:[4]
- N-deethylation to the primary amine.
- Reduction of the keto moiety to the respective alcohol.
Legal Status
As of October 2015 Ethylone is a controlled substance in China.[5]
See also
References
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Substance Details Ethylone". Retrieved 2024-01-22.
- ^ a b Lee D, Chronister CW, Hoyer J, Goldberger BA (September 2015). "Ethylone-Related Deaths: Toxicological Findings". Journal of Analytical Toxicology. 39 (7): 567–71. doi:10.1093/jat/bkv053. PMID 26025164.
- ^ Meyer MR, Wilhelm J, Peters FT, Maurer HH (June 2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry". Analytical and Bioanalytical Chemistry. 397 (3): 1225–33. doi:10.1007/s00216-010-3636-5. PMID 20333362. S2CID 21471611.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
External links
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
