| Kaempferol 3-O-β-D-rutinósido | ||
|---|---|---|
 | ||
| Nombre IUPAC | ||
|
7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-3, 5-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| General | ||
| Otros nombres |
Kaempferol 3-O-rutinoside Kaempferol 3-O-rhamnosyl-glucoside Nicotiflorine Kaempferol-3-O-β-D-glucopyranoside-7-O-α-L-rhamnopyranoside kaempferol 7-neohesperidoside | |
| Fórmula estructural |
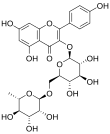 | |
| Fórmula molecular | ? | |
| Identificadores | ||
| Número CAS | 31921-42-3[1] | |
| ChEBI | 69657 | |
| ChEMBL | CHEMBL498879 | |
| ChemSpider | 4588328 | |
| PubChem | 5483905 | |
| UNII | 4056D20K3H | |
|
CC1C(C(C(C(O1)OC2C(C(C(OC2OC3=CC(=C4C(=C3)OC(=C(C4=O)O)C5=CC=C(C=C5)O)O)CO)O)O)O)O)O
| ||
| Propiedades físicas | ||
| Masa molar | 594,52 g/mol | |
| Valores en el SI y en condiciones estándar (25 ℃ y 1 atm), salvo que se indique lo contrario. | ||
Kaempferol-3-O-rutinósido con fórmula química C27H30O15, es un glucósido de flavonol de sabor amargo. Se puede aislar de los rizomas del helecho Selliguea feei.[2]
Referencias
- ↑ Número CAS
- ↑ Flavonoids and a proanthrocyanidin from rhizomes of Selliguea feei. Baek Nam-In, Kennelly E.J., Kardono L.B.S., Tsauri S., Padmawinata K., Soejarto D.D. and Kinghorn A.D., Phytochemistry, 1994, vol. 36, no2, pages 513-518, INIST 3300075
Enlaces externos
- Esta obra contiene una traducción derivada de «Kaempferol 3-O-rutinoside» de Wikipedia en inglés, publicada por sus editores bajo la Licencia de documentación libre de GNU y la Licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional.
- Kaempferol-3-O-rutinoside at www.phenol-explorer.eu
