| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| WOF4 | |
| Molar mass | 275.83 g/mol |
| Appearance | colourless crystals[1] |
| Density | 5.07 g/cm3[2] |
| Melting point | 110[2] °C (230 °F; 383 K) |
| Boiling point | 185[2] °C (365 °F; 458 K) |
| reacts[2] | |
| Solubility | soluble in chloroform[3] sparingly soluble in carbon disulfide[3] |
| Structure | |
| monoclinic | |
| Related compounds | |
Other anions
|
Tungsten(VI) oxytetrachloride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
Structure
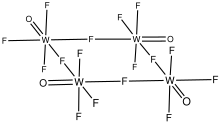
As confirmed by X-ray crystallography, WOF4 crystallizes as a tetramer. The oxides are terminal, and four of the fluorides are bridging.[4] Its structure is similar to those for niobium pentafluoride and tantalum pentafluoride. In contrast, molybdenum oxytetrafluoride adopts a polymeric structure, although again the fluorides bridge and the oxides are terminal.[5]
In the gas state, this molecule is a monomer.[6] It can form complexes with acetonitrile and other compounds.[7][8]
Preparation
Tungsten(VI) oxytetrafluoride can be synthesized by the reaction of fluorine and tungsten trioxide.[4]
It can also be obtained by treating tungsten with a mixture of oxygen and fluorine at high temperatures.[1] Partial hydrolysis of tungsten hexafluoride will also produce WOF4.[9]
- WF6 + H2O → WOF4 + 2 HF
The reaction of tungsten(VI) oxytetrachloride and hydrogen fluoride will also produce WOF4.[3]
- WOCl4 + 4HF → WOF4 + 4HCl
WOF4 can also prepared by the reaction of lead(II) fluoride and tungsten trioxide at 700 °C.[3]
- 2PbF2 + WO3 → WOF4 + 2PbO
Tungsten(VI) oxytetrafluoride hydrolyzes into tungstic acid.[1][9]
- WOF4 + 2 H2O → WO3 + 4 HF
References
- ^ a b c Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of inorganic compounds. Boca Raton: CRC Press. p. 428. ISBN 0-8493-8671-3. OCLC 32347397.
- ^ a b c d Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2017). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. Boca Raton, Florida. p. 104. ISBN 978-1-4987-5429-3. OCLC 957751024.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b c d Lassner, Erik; Schubert, Wolf-Dieter (1999). Tungsten : Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds. Boston, MA. p. 168. ISBN 1-4615-4907-8. OCLC 1113605323.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b Turnbull, Douglas; Chaudhary, Praveen; Leenstra, Dakota; Hazendonk, Paul; Wetmore, Stacey D.; Gerken, Michael (2020). "Reactions of Molybdenum and Tungsten Oxide Tetrafluoride with Sulfur(IV) Lewis Bases: Structure and Bonding in [WOF4]4, MOF4(OSO), and [SF3][M2O2F9] (M = Mo, W)". Inorganic Chemistry. 59 (23): 17544–17554. doi:10.1021/acs.inorgchem.0c02783. PMID 33200611. S2CID 226989898.
- ^ Edwards, A. J.; Steventon, B. R. (1968). "Fluoride crystal structures. Part II. Molybdenum oxide tetrafluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 2503. doi:10.1039/j19680002503.
- ^ Johnson, B. F. G. (1976). Inorganic chemistry of the transition elements. Volume 4, A review of the literature published between October 1973 and September 1974. London: Chemical Society. p. 138. ISBN 978-1-84755-645-5. OCLC 820579758.
- ^ Levason, William; Reid, Gillian; Zhang, Wenjian (2016). "Coordination complexes of the tungsten(VI) oxide fluorides WOF4 and WO2F2 with neutral oxygen- and nitrogen-donor ligands". Journal of Fluorine Chemistry. Elsevier BV. 184: 50–57. doi:10.1016/j.jfluchem.2016.02.003. ISSN 0022-1139.
- ^ Arnaudet, Lucile; Bougon, Roland; Charpin, Pierrette; Isabey, Jacques; Lance, Monique; Nierlich, Martine; Vigner, Julien (1989). "Preparation, characterization, and crystal structure of the adducts WOF4.nC5H5N (n = 1, 2)". Inorganic Chemistry. American Chemical Society (ACS). 28 (2): 257–262. doi:10.1021/ic00301a020. ISSN 0020-1669.
- ^ a b Mendicino, L.; Electrochemical Society. Dielectric Science and Technology Division; Electrochemical Society. Meeting; Symposium on Environmental Issues with Materials and Processes in the Electronics and Semiconductor Industries (2001). Environmental issues with materials and processes for the electronics and semiconductor industries : proceedings of the fourth international symposium. Pennington, NJ: Electrochemical Society. p. 180. ISBN 1-56677-312-1. OCLC 48710248.
