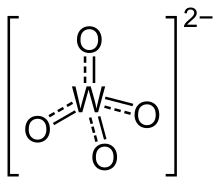
In chemistry, a tungstate is a compound that contains an oxyanion of tungsten or is a mixed oxide containing tungsten. The simplest tungstate ion is WO2−4, "orthotungstate".[1] Many other tungstates belong to a large group of polyatomic ions that are termed polyoxometalates, ("POMs"), and specifically termed isopolyoxometalates as they contain, along with oxygen and maybe hydrogen, only one other element. Almost all useful tungsten ores are tungstates.[2]
YouTube Encyclopedic
-
1/3Views:253 568506 248551
-
Making Caesium Tungstate; Heavy Liquid
-
Making Clerici's Solution; The Heaviest Water Based Liquid?
-
Mod-22 Lec-22 Molybdenum Enzymes - I
Transcription
Structures
Orthotungstates feature tetrahedral W(VI) centres with short W–O distances of 1.79 Å. Structurally, they resemble sulfates. Six-coordinate, octahedral tungsten dominates in the polyoxotungstates. In these compounds, the W–O distances are elongated.[1]
Some examples of tungstate ions:[3]
- HWO−4 (hydrogentungstate)[3]
- polymeric W2O2−7 ions of various structures in Na2W2O7, Li2W2O7 and Ag2W2O7[4]
- [W7O24]6− (paratungstate A)[3]
- [W10O32]4− (tungstate Y)[5]
- [H2W12O42]10− (paratungstate B) [3]
- α-[H2W12O40]6− (metatungstate)[5]
- β-[H2W12O40]6− (tungstate X)[5]
See the tungstates category for a list of tungstates.
Occurrence
Tungstates occur naturally with molybdates. Scheelite, the mineral calcium tungstate, often contains a small amount of molybdate. Wolframite is manganese and iron tungstate, and all these are valuable sources of tungsten. Powellite is a mineral form of calcium molybdate containing a small amount of tungstate.
Reactions
Solutions of tungstates, like those of molybdates, give intensely blue solutions of complex tungstate(V,VI) analogous to the molybdenum blues when reduced by most organic materials.[1]
Unlike chromate, tungstate is not a good oxidizer, but like chromate, solutions of tungstate condense to give the isopolytungstates upon acidification.
References
- ^ a b c Egon Wiberg, Arnold Frederick Holleman (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5.
- ^ Lassner, Erik; Schubert, Wolf-Dieter; Lüderitz, Eberhard; Wolf, Hans Uwe (2005). "Tungsten, Tungsten Alloys, and Tungsten Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_229.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ a b c Jon A. McCleverty, N. G. Connelly,Nomenclature of inorganic chemistry II: recommendations 2000, International Union of Pure and Applied Chemistry Commission on the Nomenclature of Inorganic Chemistry, Published by Royal Society of Chemistry, 2001, ISBN 0-85404-487-6
