| |||

| |||
| Names | |||
|---|---|---|---|
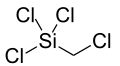
| Preferred IUPAC name
Trichloro(chloromethyl)silane | |||
| Other names
(Chloromethyl)trichlorosilane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1811640 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.014.834 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3389 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2Cl4Si | |||
| Molar mass | 183.91 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Boiling point | 118 °C (244 °F; 391 K) | ||
| Reacts with water | |||
| Solubility | Reacts with alcohols Soluble in chloroform, benzene, THF | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable, hydrolyzes to release HCl | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trichloro(chloromethyl)silane is a compound with formula Si(CH2Cl)Cl3.