Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
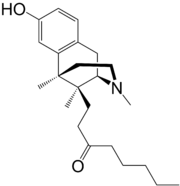
| Formula | C23H35NO2 |
| Molar mass | 357.538 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tonazocine (WIN-42,156) is an opioid analgesic of the benzomorphan family which made it to phase II clinical trials for the treatment of postoperative pain,[1] but development was apparently ceased and ultimately it was never marketed. Tonazocine is a partial agonist at both the mu-opioid and delta-opioid receptors, but acting more like an antagonist at the former and more like an agonist at the latter.[2][3] It lacks most of the side effects of other opioids such as adverse effects on the cardiovascular system and respiratory depression, but it can cause sedation (although to a lesser degree of typical opioids), and in some patients it may induce hallucinations (probably via binding to and activating the κ-opioid receptor).[4]
See also
References
- ^ American Chemical Society. Division of Medicinal Chemistry (1990). Annual Reports in Medicinal Chemistry. Academic Press. p. 12. ISBN 978-0-12-040525-1. Retrieved 30 November 2011.
- ^ Ward SJ, Pierson AK, Michne WF (February 1985). "Pharmacological profiles of tonazocine (Win 42156) and zenazocine (Win 42964)". Neuropeptides. 5 (4–6): 375–8. doi:10.1016/0143-4179(85)90032-0. PMID 2860595. S2CID 20674308.
- ^ Hudzik TJ, Howell A, Payza K, Cross AJ (May 2000). "Antiparkinson potential of delta-opioid receptor agonists". European Journal of Pharmacology. 396 (2–3): 101–7. doi:10.1016/S0014-2999(00)00209-0. PMID 10822062.
- ^ Aronson, Jeffrey K. (30 November 2009). Meyler's Side Effects of Analgesics and Anti-inflammatory Drugs. Elsevier. p. 154. ISBN 978-0-444-53273-2. Retrieved 30 November 2011.
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| μ-opioid (MOR) |
| ||||
|---|---|---|---|---|---|
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
