 | |
| Clinical data | |
|---|---|
| Trade names | Tolevamer |
| Pharmacokinetic data | |
| Bioavailability | None |
| Metabolism | None |
| Excretion | Faeces (100%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.553 |
| Chemical and physical data | |
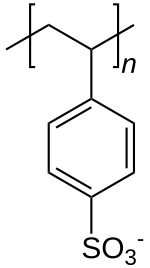
| Formula | [C8H7SO3−] n |
Tolevamer is a medication developed to combat Clostridium difficile associated diarrhea.[1] It is a potassium sodium polystyrene sulfonate. It was never marketed.
Mechanism of action
Tolevamer was designed to bind the enterotoxins of Clostridium difficile. Since it has no antibiotic properties, it does not harm the gut flora. Early studies used the sodium salt, but it was soon replaced with the potassium sodium salt to prevent hypokalaemia, which is often associated with diarrhea.[2][3]
History
Termination of development
In early 2008, a noninferiority study versus vancomycin or metronidazole for Clostridium difficile associated diarrhea (CDAD) found that about half of the patients in the tolevamer group did not complete the treatment, versus 25% in the vancomycin and 29% in the metronidazole groups. CDAD recurrence in patients reaching clinical success was reduced significantly by tolevamer (6% recurrence rate), vancomycin (18%) and metronidazole (19%). However, the good result of tolevamer is partly due to the high drop-out rate in this group. Since tolevamer did not reach its primary endpoint in this study, its development was halted.[4]
References
- ^ Hinkson PL, Dinardo C, DeCiero D, Klinger JD, Barker RH (June 2008). "Tolevamer, an anionic polymer, neutralizes toxins produced by the BI/027 strains of Clostridium difficile". Antimicrobial Agents and Chemotherapy. 52 (6): 2190–5. doi:10.1128/AAC.00041-08. PMC 2415796. PMID 18391047.
- ^ Spreitzer H (September 24, 2007). "Neue Wirkstoffe - Tolevamer". Österreichische Apothekerzeitung (in German) (20/2007): 955.
- ^ Wang Y, Serradell N, Rosa E, Bolos J (2007). "Tolevamer Potassium Sodium". Drugs of the Future. 32 (6): 501–505. doi:10.1358/dof.2007.032.06.1108513.
- ^ Berrie C (24 April 2008). "Tolevamer Less Effective Than Standard Therapies for C difficile–Associated Diarrhea". Medscape.com.
