 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tolinase |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682482 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | metabolized in the liver to active metabolites |
| Elimination half-life | 7 hours |
| Excretion | Renal (85%) and fecal (7%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.013.262 |
| Chemical and physical data | |
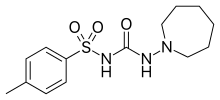
| Formula | C14H21N3O3S |
| Molar mass | 311.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tolazamide is an oral blood glucose lowering drug used for people with Type 2 diabetes. It is part of the sulfonylurea family (ATC A10BB).
YouTube Encyclopedic
-
1/5Views:48914 85813 1777 794361
-
Hypoglycemics
-
Oral Antidiabetic Drugs Classification
-
SYNTHESIS OF CHLORTHIAZIDE | PHARMACEUTICAL CHEMISTRY
-
#42 Oral Hypoglycemic Drugs in Tamil | Antidiabetic Drugs
-
Anti Diabetic Agent II Medicinal Chemistry II TYPE 1 IDDM II TYPE 2 NIDDM II #METFORMIN II #GLIPTINE
Transcription
Synthesis

<i>para</i>-Toluenesulfonamide is converted to its carbamate with ethyl chloroformate in the presence of a base. Heating that intermediate with 1-amino-azepane leads to the displacement of the ethoxy group and the formation of tolazemide:[1]
Azepane proper would lead to [13078-23-4].
References
External links
- "Tolazamide". Medline Plus. U.S. National Library of Medicine.
