| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methyl-1-(propan-2-yl)cyclohex-3-en-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.396 |
| MeSH | terpinenol-4 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.253 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Terpinen-4-ol is an isomer of terpineol with the chemical formula C10H18O. A primary constituent of tea tree oil,[1] it is obtained as an extract from the leaves, branches, and bark of Melaleuca alternifolia Cheel.[2][3][4] Despite considerable basic and preliminary clinical research of terpinen-4-ol and tea tree oil, its biological properties and potential for clinical uses have not been established as of 2019.[2] It may be a factor in the contact dermatitis of tea tree oil when used topically.[2][3]
Terpinen-4-ol occurs in Juniperus communis and is thought to be the reason why this wood is highly resistant to rot.[citation needed]
YouTube Encyclopedic
-
1/1Views:328
-
Propiedades medicinales La Mejorana
Transcription
Synthesis
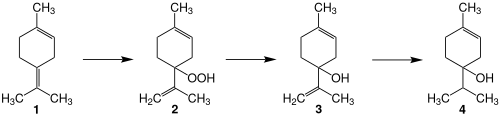
Terpinen-4-ol can be synthesized from terpinolene (1) by photooxidation, reduction of the resulting hydroperoxide (2), and selective hydrogenation of the terminal double bond in 3.
References
- ^ "Tea tree oil". Drugs and Lactation Database (LactMed), National Library of Medicine, US National Institutes of Health. 3 December 2018. Retrieved 31 July 2019.
- ^ a b c "Tea tree oil". Drugs.com. 17 June 2019. Retrieved 31 July 2019.
- ^ a b de Groot, Anton C.; Schmidt, Erich (13 May 2016). "Tea tree oil: contact allergy and chemical composition". Contact Dermatitis. 75 (3): 129–143. doi:10.1111/cod.12591. ISSN 0105-1873. PMID 27173437.
- ^ Hammer, K. A.; Carson, C. F.; Riley, T. V. (2012). "Effects of Melaleuca alternifolia (Tea Tree) Essential Oil and the Major Monoterpene Component Terpinen-4-ol on the Development of Single- and Multistep Antibiotic Resistance and Antimicrobial Susceptibility". Antimicrobial Agents and Chemotherapy. 56 (2): 909–915. doi:10.1128/AAC.05741-11. PMC 3264233. PMID 22083482.
{{cite journal}}: CS1 maint: multiple names: authors list (link)