| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.070 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TeBr4 | |
| Molar mass | 447.22 g/mol |
| Appearance | yellow-orange crystals |
| Density | 4.3 g/cm3, solid |
| Melting point | 388 °C (730 °F; 661 K)[1] |
| Boiling point | decomposes at 420 °C (788 °F; 693 K) |
| Structure | |
| monoclinic | |
| Hazards | |
| GHS labelling:[2] | |
 
| |
| Danger | |
| H301, H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Related compounds | |
Other anions
|
Tellurium tetrafluoride Tellurium tetrachloride Tellurium tetraiodide |
Other cations
|
Selenium tetrabromide |
Related compounds
|
Ditellurium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
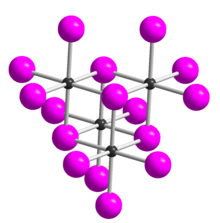
Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4.[3] It can be made by reacting bromine and tellurium.[4] In the vapour TeBr4 dissociates:[3]
- TeBr4 → TeBr2 + Br2
It is a conductor when molten, dissociating into the ions TeBr3+ and Br−. When dissolved in benzene and toluene, TeBr4 is present as the unionized tetramer Te4Br16.[3] In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
- TeBr4 + 2CH3CN → (CH3CN)2TeBr3+ + Br−
YouTube Encyclopedic
-
1/3Views:18 98226 4781 385
-
TeF4 Lewis Structure: How to Draw the Lewis Structure for TeF4 (Tellurium Tetrafluoride)
-
TeCl4 Lewis Structure: How to Draw the Lewis Structure for TeCl4 (Tellurium Tetrachloride)
-
Tellurium Chemistry: Tellurium in HNO3 [2x]
Transcription
References
- ^ Thermochemical Data of Elements and Compounds", M. Binnewies, E. Milke, Wiley-VCH, 2002, ISBN 3-527-30524-6
- ^ "C&L Inventory". echa.europa.eu.
- ^ a b c Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
