 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H22N2O |
| Molar mass | 342.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
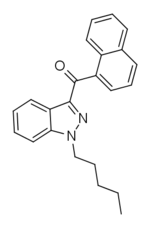
THJ-018 (SGT-17) is a synthetic cannabinoid that is the indazole analogue of JWH-018 and has been sold online as a designer drug.[1][2]
YouTube Encyclopedic
-
1/4Views:1 2033425 02686 309
-
[한글자막]도묘필기 중계 비하인드009-샤오거의 첫낙하촬영The Lost Tomb Reboot Xiaoge's the First Gyro Drop BTS 9 黄俊捷
-
Synthetic cannabinoids | Wikipedia audio article
-
AM-2201 Available - Retail & Wholesale
-
الدرس الأول : المصفوفة | الوحده 2 | رياضيات الصف الثاني عشر أدبي وشرعي | توجيهي
Transcription
Pharmacology
THJ-018 acts as a full agonist with a binding affinity of 5.84 nM at CB1 and 4.57 nM at CB2 cannabinoid receptors.[3]
Legality
THJ-018 is an Anlage II controlled drug in Germany.[4] It is also banned in Sweden.[5]
See also
References
- ^ "THJ-018". Cayman Chemical. Retrieved 9 July 2015.
- ^ Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA (January 2016). "High-Resolution Mass Spectrometry for Characterizing the Metabolism of Synthetic Cannabinoid THJ-018 and Its 5-Fluoro Analog THJ-2201 after Incubation in Human Hepatocytes". Clinical Chemistry. 62 (1): 157–69. doi:10.1373/clinchem.2015.243535. PMID 26430074.
- ^ Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE (2016). "Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice". Forensic Toxicology. 34 (2): 329–343. doi:10.1007/s11419-016-0320-2. PMC 4929166. PMID 27429655.
- ^ "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)" (in German). Retrieved 9 July 2015.
- ^ "Cannabinoider föreslås bli klassade som hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. Retrieved 9 July 2015.
