 | |
| Clinical data | |
|---|---|
| Other names | ACH-1625 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
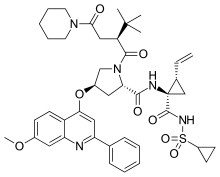
| Formula | C43H53N5O8S |
| Molar mass | 799.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sovaprevir (codenamed ACH-1625) is an experimental drug designed to treat the hepatitis C virus. It is under development by Achillion Pharmaceuticals. It acts as a NS3/4A inhibitor.[1] Sovaprevir received fast track status from the U.S. Food and Drug Administration in 2012.[2]
YouTube Encyclopedic
-
1/1Views:957
-
Conversations from CROI 2014: Dr. Doug Dieterich
Transcription
At CROI 2014, Dr. Ronald Valdiserri spoke with Dr. Doug Dieterich of Mount Sinai Hospital. Valdiserri: Hello, this is Dr. Ron Valdiserri and I'm reporting from the 2014 Conference on Retrovirus and Opportunistic Infections in Boston, and we're very honored to have with us, Dr. Doug Dieterich, who is a liver specialist from Mount Sinai Hospital in New York. And actually, Dr. Dietrich has presented some very groundbreaking information at this meeting, but what I want to ask you, Dr. Dieterich, if I might -- what would you want to tell primary care providers about what's happening in the field of hepatitis C treatment today? Dieterich: Well I think there's a couple of take home messages that are really important for the primary care people to understand. Number one -- the size of the epidemic as the CDC and the USPTF have pointed out. It may be as many as five million people or more in the US, and 75% of them don't know they have it, so we need to get them tested. And the primary care people have a really huge role in testing those people for hepatitis C. Valdiserri: And we know, if we could pause now, you might want to share with the viewers that we now have both the CDC and the US Preventive Services Task Force saying the same thing about screening for hepatitis C. What is that message? Dieterich: Right. The message is screen all baby boomers born between 1945 and 1965. Valdiserri: Just to be clear, this means the provider does not have to elicit a risk history. This is based on age. Dieterich: Absolutely. Based on age. And frankly, I don't even ask about risks. It's like, I don't care how you got it, because some people feel guilty, and some don't want to tell you the things they did back in the Woodstock generation that got them hepatitis C. I don't care -- who cares? It's like, they have it, they have it. It's like hypertension, it's not their fault. Valdiserri: You want to take care of it. You want to diagnose it and take care of it. Dieterich: We'll that's the other point, actually. We can cure it. We don't just take care of it, Ron. We cure it. So when we cure it, it's gone. And the liver disease in the vast majority of patients gets better. So that's a huge, huge thing. Valdiserri: That's a huge difference with HIV or other types of infections that we can treat, but not cure. Dieterich: Right. And, actually, it's not just the liver disease anymore. Actually, and that's another message that the primary care treaters really, I think should understand. There's been three big studies published now -- The VA study with 400,000 patients, the Amsterdam study, and the Reveal study from Taiwan -- all showed all cause mortality, that is diabetes, heart disease, cancer, everything else, was doubled in patients who had hepatitis C. So it's not just liver disease, it's a chronic and inflammatory condition that we need to cure and get rid of, and if we can do that we can bring mortality back down to that of the baseline. Valdiserri: So I know that you are a world class expert in these issues. I'm going to ask you a very basic question that I'd like to ask you to answer for the audience. Let's say 2 years ago, even, a lot of providers shyed away from treating hepatitis C because of the need to use interferon. Can you talk generally about where the field is headed, particularly in terms of interferon-free treatments? And why that's so important, not only for providers, but for patients. Dieterich: Yeah, well actually, it's important for everybody. Even in pairs, because it turns out to be cheaper as well. Yes, as of December 6, we got two new drugs approved in the US. Two weeks before that simeprevir, and December 6, sovaprevir -- both of them are one pill, once a day. sovaprevir is approved for genotype two and three. Just one pill, once a day, with ribavirin. And it cures 80-90% of genotype 2, 3 with either 12 or 24 weeks of treatment -- no injections, no interferon, and virtually no side-effects. That's extraordinary from where we've been with the interferon and of course, our last level of drugs with telaprevir and boceprevir and the side effects that we had with those. For genotypes one and four -- the harder ones -- it's recommended in the label to use it with interferon for 12 weeks, which is not so bad -- it's doable -- but there's new guidelines put out by the ASLD, IDSA, and ISUSA together that suggest we can use an off label -- and we are actually using an off-label quite a bit now with simeprevir-- so one pill of simeprevir and one pill of sovaprevir, with or without ribavirin, you don't necessarily need it -- cures somewhere between 90-100% of people who take that for 12 weeks, and that's extraordinary, so I'm almost not using interferon really at all -- only for a rare genotype four, so it's really hard to get my patients in New York to take interferon for any reason, frankly, at this point. On the horizon, in the fall, we have two extraordinary compounds coming -- the Gilead fixed-dose combination -- one pill, once a day -- for all genotypes -- oh right, that's just genotype one. And the Ab-V combo which is three pills a day, which cures, we just saw that presentation, 99 percent of people in 12 weeks. Valdiserri: So the short version of that is that the treatments are getting a lot better, and they're getting shorter, and easier to tolerate with virtually zero side effects. So what would you tell, I'm going to ask you now, you talk to patients day in and day out in your job. What would you tell to persons who might be watching this blog, who perhaps are living with hepatitis C and haven't taken advantage of these treatment options? What would you say to them? What kind of conversation should they have with their provider? Dieterich: Well you know what I typically say to the patients, when they come in holding their blood test in their hand -- a little teary-eyed, and gloomy and depressed. What I tell them is it's a really good time to have hepatitis C. If you have to have it, now is the time to have it because we have these marvelous drugs - we can treat you with right now, without side effects, if necessary. If there's no rush, we can wait until the fall, when it's going to be even easier actually, and more likely to succeed. And this time next year, we'll have another round of drugs that are available. So it's a really good time to have hepatitis C. Valdiserri: One last question. This is something that in the field of HIV, we've heard a lot about for the last five years and that's treatment as prevention. With hepatitis C I know there have been several studies that have looked at this in terms of modelling it, in other words they're not actual real experiments, but people saying that if we did this maybe that would happen, but namely the issue of if we can treat, especially in the people that inject drugs, but if we can successfully treat hepatitis C, not only are we helping that patient live a healthier life, but we might actually interrupt transmission. I wonder if you have any initial thoughts on that? I know we're at the beginning of that in the hepatitis field. Dieterich: With hepatitis it's a little more complicated than with HIV because you can get it again, and we have seen reinfections. And frankly the other group that's subject -- Valdiserri: So after cure, you can get it again, you can be re infected if you're re-exposed. Dieterich: Yes, that does not provide lifelong immunity. Valdiserri: Good important point for people to know. Dieterich: Yes exactly, because you can't say "Yeah now I'm fine I can go out and do whatever I want" You can't do that. The other group that's actually getting a lot of acute hepatitis C reinfection is the men who have sex with men. Actually the acute hep C in men who have sex with men who are practicing unsafe sex again -- it's back. We just came from a session on that right now. So that's a big issue. If we treated all those men -- absolutely all of them -- we could eradicate the virus -- but that would be a big hurdle. Valdiserri: You just mentioned two or three really important prevention messages for people who are watching this. Number one -- that being exposed to hep C and cured, does not confer immunity. A very important prevention message. And the other one is that we know that from the CDC, we know that most cases of hepatitis C in the United States are related to exposure to infected blood. Whether that's from a past or current history of injection drug use or exposure in a healthcare setting, or intranasal -- very good point -- using instruments to take cocaine. As you know historically, sexual transmission was always played down for hepatitis C, because it wasn't a very frequent cause of transmission -- but as you pointed out, at this meeting we're hearing that for some populations, men who have sex with men, there's more concern about the potential for sexual transmission. True? Dieterich: You're absolutely right. You're absolutely correct. Heterosexual transmission is virtually zero through heterosexual vaginal sex. Anal sex is another issue and that's the issue for men who have sex with men. But it's not even just anal sex -- its toys, its rough sex, its booty bumping, its other things that go on. And actually, it's methamphetamine at the same time. So sometimes they're snorting it, sometimes they're injecting the methamphetamine, along with all the other sexual practices. Valdiserri: So these are all unhealthy practices? Dieterich: They're all very unsafe practices. Valdiserri: So we definitely want to focus on the importance of prevention as well. Let me just say that it's been a pleasure talking with you Dr. Dieterich. Persons born between 1945-1965 should get a one-time HCV test. New, effective treatments can cure hepatitis C in a shorter time, with fewer side effects. Join the conversation: AIDS.gov, blog.AIDS.gov, twitter.com/aidsgov
References
- ^ De Clercq E (June 2014). "Current race in the development of DAAs (direct-acting antivirals) against HCV". Biochemical Pharmacology. 89 (4): 441–52. doi:10.1016/j.bcp.2014.04.005. PMID 24735613.
- ^ "Sovaprevir HCV NS3/4A Protease Inhibitor". Achillion.
