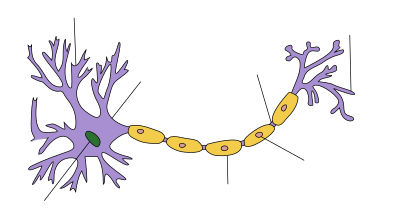
In neuroscience, saltatory conduction (from Latin saltus 'leap, jump') is the propagation of action potentials along myelinated axons from one node of Ranvier to the next node, increasing the conduction velocity of action potentials. The uninsulated nodes of Ranvier are the only places along the axon where ions are exchanged across the axon membrane, regenerating the action potential between regions of the axon that are insulated by myelin, unlike electrical conduction in a simple circuit.
YouTube Encyclopedic
-
1/3Views:278 93659 40210 207
-
Saltatory conduction in neurons | Human anatomy and physiology | Health & Medicine | Khan Academy
-
Myelination and Saltatory Conduction
-
Myelin and saltatory conduction | Action Potentials in Neurons
Transcription
Now that we know how a signal can spread through a neuron, through an electrotonic potential and action potential and combinations of the two, let's put it all together by looking again at the structure of a neuron, the anatomy of a neuron, and thinking about why it has that anatomy and how it all can work. So we've already talked about the dendrites as being where the neuron can be stimulated from multiple inputs. If we're in the brain, these dendrites might be near the terminal ends of axons of other neurons. If we're some type of sensory cell, these dendrites could be stimulated by some type of sensory input. But let's just say, for the sake of argument, they are stimulated in some way. And because they're stimulated in some way, it allows positive ions to flood into the neuron from the outside. As we know, there's a potential difference. It's more negative inside of the neuron than outside of the neuron. And so if a channel gets opened up because of some stimulus, that would allow positive ions to flow in. And the primary positive ions we've been talking about are the sodium ions. Maybe this is some type of sodium gate that gets opened up because of this stimulus. So when that happens, you will have electrotonic spread. You will have an electrotonic potential being spread. So let's say that we had a voltmeter right here on the axon hillock. It's kind of the hill that leads to the axon right over here. So what you might see happening after some amount of time-- so let me draw. So let's say this is our voltage in millivolts across the membrane-- our voltage difference, I should say. This is the passage of time. Let's say the stimulus happens at time 0. But right at time 0, we haven't really noticed it with our voltmeter. Our voltage right across the membrane right over there is at that equilibrium, negative 70 millivolts. But after some small amount of time, this electrotonic potential has gotten to this point, because all of these positive charges are trying to get away from each other. It's gotten to that point. And you might see a bump in the voltage-- in the voltage difference, I guess I should say. This thing might go up. So it might look something like that. Now, that by itself might not be-- we might have gotten the voltage difference low enough, I guess we could say. Or we might not have gotten the voltage inside of the cell positive enough in order to trigger the voltage-gated ion channels. And so maybe nothing happens. Maybe this right over here, this is negative 55 millivolts. And so that's what you have to get the voltage up to, the voltage difference up to, in order to trigger the ion channels right over there. So those are the sodium channels to get positive charge in. Here's the potassium channels to get the positive charge out. The axon hillock has a ton of these, because these are really there. Once they get triggered, they can trigger an impulse that can then go down the entire axon, and maybe stimulate other things, maybe in the brain or whatever else this neuron might be connected to. So maybe that stimulus by itself didn't trigger it. But let's say that there's another stimulus that happens right at the same time, or around the same time. And that happens. And on its own, that might have caused a similar type of bump right over here. But when you add the two together and they're happening at the same time, their combined bumps are enough to trigger an action potential in the hillock, or a series of action potentials in the hillock. And so then, you really have, essentially, fired the neuron. So now all sorts of positive charge gets flushed into the neuron. And then purely through electrotonic spread, you will have this electrotonic potential spread down the axon. Now, this is the interesting part, because we can think a little bit about, what is the best way for an axon to be designed? In general, if you're trying to transfer a current, the ideal thing to do is, the thing that you're transferring the current down should conduct really well. Or you could say it has low resistance. But you want it to be surrounded by an insulator. You want it to be surrounded. So if this was a cross section, you want it to be surrounded by an insulator that has high resistance. And the reason is because you don't want the potential to leak across your membrane-- high resistance right over here. If you didn't have something high resistance around it, your current would actually go slower. This is true if you're just dealing with electronics. If you just had a bunch of copper wires on one side, and you had some copper wires that were surrounded by a really good insulator, a really good resistor-- for example, plastic or rubber of some kind. The current is actually going to have less energy loss. It's going to travel faster when it's surrounded by an insulator. So you might say, OK, well gee. The best thing to do would be to surround this entire axon with a good insulator. And for the most part, that is true. It is surrounded by a good insulator. That is what the myelin sheath is. So let's say we want to surround this whole thing with just one big grouping of Schwann's cells, so one big myelin sheath-- which is a good insulator. It does not conduct current well. So this right over here is just one big myelin sheath right over here. Now, what's the problem with this? Well, if this axon is really long-- and let's say, you know, you're a dinosaur or something. And you're trying to go up your neck, and your neck is 20 feet long. Or even a human being, we're a reasonable size. And you're going several feet, or even whatever, you want to go a reasonable distance purely with electrotonic spread, your signal, remember, it dissipates. Your signal is going to be really weak right over here. You're going to have a weak signal on the other end. It might not be even strong enough to make anything interesting happen at these terminals, which wouldn't be strong enough to trigger, maybe, other neurons, or whatever else might need to happen at this other end. So then you say, OK, well then why don't we try to boost the signal? Well, how would you boost the signal? You say, OK. I like having this myelin sheath. But why don't we put gaps in the myelin sheath every so often? And then those gaps would allow the membrane to interface with the outside. And in those areas, we could put some voltage-gated channels that can release action potentials, in order to essentially boost the signal. And that's is exactly what the anatomy of a typical neuron is like. So instead of just one big insulating sheath like this, it would-- let me make some gaps here. Whoops, I'm going to do that in black. So actually, let me just draw it like this. Let me just erase this. So clear, and let me clear this. That's good enough. And so what we could do is we could put gaps in it right over here where the axon, the axonal membrane itself can interface with its surroundings. And of course, we know we call those gaps the nodes of Ranvier, or Ran-Veer. I'm not really sure how to pronounce it. So let me put those gaps in here. So you put those gaps in here, so these are the myelin sheath. And this right over here is a node of Ranvier. These are nodes of Ran-Veer, or Ranvier. And right in those little nodes, right in those nodes, right where the myelin sheath isn't, we can put these voltage-gated channels to essentially boost the signal. If the signal had to go electrotonically all the way over here, it'd be very weak. It's going to dissipate as it goes down, but it could be just strong enough right at this point in order to trigger these voltage-gated channels, in order to essentially boost the signal again, in order to trigger an action potential, boost the signal. And now the signal is boosted, it'll dissipate, dissipate, dissipate, boost. And it'll boost right over here again. And then it'll dissipate, dissipate, dissipate, and boost. Dissipate, dissipate, boost. And so by having this combination, you want the myelin sheath. You want the insulator in order to keep the transmission of the current to fast, in order to have minimal energy loss. But you do need these areas where the myelin sheath isn't in order to boost the signal, in order for the action potentials to get triggered, and so your signal can keep being-- well, I guess keep being amplified, if we wanted to talk in kind of electrical engineering speak. And this type of conduction, where the signal just keeps boosting, and if you were just to superficially observe it, it looks like the signal is almost jumping. It gets triggered here, then it gets triggered, here then it gets triggered here, then it gets triggered here, then it gets triggered here. This is called saltatory conduction. It comes from the Latin word saltare-- once again, I don't know how to pronounce. My Latin isn't too good. But it comes from the Latin word saltare, which means to jump around or to hop around. And that's because it looks like the signal is hopping around. But that's not exactly what's happening. The signal is traveling passively through. It gets triggered here in the axon hillock. Then it travels passively through electrotonic spread. And then it gets boosted. And you have the myelin sheath around it to make sure it goes as fast as possible, and you get very little loss of signal. And then it gets boosted at the nodes of Ranvier, because it triggers these voltage-gated channels again. That triggers an action potential. And then your signal gets boosted, and then it dissipates-- boosted, dissipates, boosted, dissipates, boosted, dissipates. Maybe it could even get boosted again. And then it can trigger whatever else it has to trigger.
Mechanism
Myelinated axons only allow action potentials to occur at the unmyelinated nodes of Ranvier that occur between the myelinated internodes. It is by this restriction that saltatory conduction propagates an action potential along the axon of a neuron at rates significantly higher than would be possible in unmyelinated axons (150 m/s compared to 0.5 to 10 m/s).[1] As sodium rushes into the node it creates an electrical force which pushes on the ions already inside the axon. This rapid conduction of electrical signal reaches the next node and creates another action potential, thus refreshing the signal. In this manner, saltatory conduction allows electrical nerve signals to be propagated long distances at high rates without any degradation of the signal. Although the action potential appears to jump along the axon, this phenomenon is actually just the rapid conduction of the signal inside the myelinated portion of the axon. If the entire surface of an axon were insulated, action potentials could not be regenerated along the axon resulting in signal degradation.
Energy efficiency
In addition to increasing the speed of the nerve impulse, the myelin sheath helps in reducing energy expenditure over the axon membrane as a whole, because the amount of sodium and potassium ions that need to be pumped to bring the concentrations back to the resting state following each action potential is decreased.[2]
Distribution
Saltatory conduction occurs widely in the myelinated nerve fibers of vertebrates, but was later discovered in a pair of medial myelinated giant fibers of Fenneropenaeus chinensis and Marsupenaeus japonicus shrimp,[3][4][5] as well as in a median giant fiber of an earthworm.[6] Saltatory conduction has also been found in the small- and medium-sized myelinated fibers of Penaeus shrimp.[7]
See also
- Bioelectrochemistry
- Cable theory
- Electrophysiology
- Ephaptic coupling – Form of nervous system communication
- GHK current equation – Expression of the ionic flux across a cell membrane
- Goldman equation – Generalization of the Nernst equation for the membrane potential
- Hindmarsh–Rose model – Of the spiking-bursting behavior of a neuron
- Hodgkin–Huxley model – Describes how neurons transmit electric signals
- Neurotransmission – Impulse transmission between neurons
- Patch clamp – Laboratory technique in electrophysiology
- Quantitative models of the action potential
- Myelination – Fatty substance that surrounds nerve cell axons to insulate them and increase transmission speed
References
- ^ Purves D, Augustine GJ, Fitzpatrick D (2001). "Increased Conduction Velocity as a Result of Myelination". Neuroscience (2nd ed.). Sunderland (MA): Sinauer Associates.
- ^ Tamarkin D. "Saltatory Conduction of APs". Archived from the original on 30 October 2014. Retrieved 6 May 2014.
- ^ Hsu K, Tan TP, Chen FS (August 1964). "On the excitation and saltatory conduction in the giant fiber of shrimp (Penaeus orientalis)". Proceedings of the 14th National Congress of the Chinese Association for Physiological Science: 7–15.
- ^ Hsu K, Tan TP, Chen FS (1975). "Saltatory conduction in the myelinated giant fiber of shrimp (Penaeus orientalis)". KexueTongbao. 20: 380–382.
- ^ Kusano K, LaVail MM (August 1971). "Impulse conduction in the shrimp medullated giant fiber with special reference to the structure of functionally excitable areas". The Journal of Comparative Neurology. 142 (4): 481–94. doi:10.1002/cne.901420406. PMID 5111883. S2CID 33273673.
- ^ Günther J (August 1976). "Impulse conduction in the myelinated giant fibers of the earthworm. Structure and function of the dorsal nodes in the median giant fiber". The Journal of Comparative Neurology. 168 (4): 505–31. doi:10.1002/cne.901680405. PMID 939820. S2CID 11826323.
- ^ Xu K, Terakawa S (1993). "Saltatory conduction and a novel type of excitable fenestra in shrimp myelinated nerve fibers". The Japanese Journal of Physiology. 43 Suppl 1: S285-93. PMID 8271510.
Further reading
- Saladin K. "Saltatory conduction". Biology Online.
- Anatomy & physiology : the unity of form and function (6th ed.). McGraw-Hill. 2011. ISBN 978-0-07-768033-6.
External links
- Saltatory conduction - Scholarpedia
- cell biology - Why is saltatory conduction in myelinated axons faster than continuous conduction in unmyelinated axons?