 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
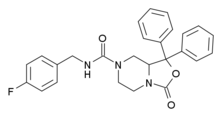
| Formula | C26H24FN3O3 |
| Molar mass | 445.494 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
SHA-68 is a drug which acts as a selective, non-peptide antagonist at the neuropeptide S receptor NPSR. In animal studies it reduced motor stereotypes, and blocks the stimulant action of neuropeptide S.[1][2]
YouTube Encyclopedic
-
1/1Views:1 116 504
-
The Sixties - The Years That Shaped a Generation (TV) [2005]
Transcription
See also
References
- ^ Fukatsu K, Nakayama Y, Tarui N, Mori M, Matsumoto H, Kurasawa O, Banno H. Bicyclic Piperazine Compound and Use Thereof. PCT Patent WO 2005/021555 A1. Published 26.08.2004
- ^ Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK (June 2008). "Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor". The Journal of Pharmacology and Experimental Therapeutics. 325 (3): 893–901. doi:10.1124/jpet.107.135103. PMC 2583099. PMID 18337476.
