| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
D-Ribose
| |||
| Systematic IUPAC name
(2R,3R,4S,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol | |||
| Other names
d-Ribose
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEMBL | |||
| ChemSpider |
| ||
| DrugBank | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties[1][2] | |||
| C5H10O5 | |||
| Molar mass | 150.13 | ||
| Appearance | White solid | ||
| Melting point | 95 °C (203 °F; 368 K) | ||
| 100 g/L (25 °C, 77 °F) | |||
Chiral rotation ([α]D)
|
−21.5° (H2O) | ||
| Related compounds | |||
Related aldopentoses
|
Arabinose Xylose Lyxose | ||
Related compounds
|
Deoxyribose | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

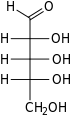
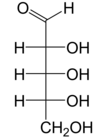
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, d-ribose, is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. l-ribose is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891.[3] It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that d-ribose was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids.[4][5][6] Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared l-ribose.[6][7]
Right: Fischer projection of the open chain forms of d- and l- ribose
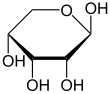
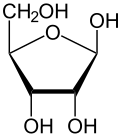
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution.[8] The name "ribose" is used in biochemistry and biology to refer to all of these forms, though more specific names for each are used when required. In its linear form, ribose can be recognised as the pentose sugar with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is associated with the systematic name (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal,[9] whilst l-ribose has its hydroxyl groups appear on the left hand side in a Fischer projection. Cyclisation of ribose occurs via hemiacetal formation due to attack on the aldehyde by the C4' hydroxyl group to produce a furanose form or by the C5' hydroxyl group to produce a pyranose form. In each case, there are two possible geometric outcomes, named as α- and β- and known as anomers, depending on the stereochemistry at the hemiacetal carbon atom (the "anomeric carbon"). At room temperature, about 76% of d-ribose is present in pyranose forms[8]: 228 (α:β = 1:2)[10] and 24% in the furanose forms[8]: 228 (α:β = 1:3),[10] with only about 0.1% of the linear form present.[11][12]
The ribonucleosides adenosine, cytidine, guanosine, and uridine are all derivatives of β-d-ribofuranose. Metabolically-important species that include phosphorylated ribose include ADP, ATP, coenzyme A,[8]: 228–229 and NADH. cAMP and cGMP serve as secondary messengers in some signaling pathways and are also ribose derivatives. The ribose moiety appears in some pharmaceutical agents, including the antibiotics neomycin and paromomycin.[10]
YouTube Encyclopedic
-
1/5Views:241 37031 27420 2822 3192 405
-
The Best Remedy for Cardiac Arrhythmias
-
Unknown Remedy for Cardiac Arrhythmias + Weak Heart | Benefits of D Ribose
-
Dr. Marcus Laux, ND - Benefits of D-RIBOSE
-
Can Ribose Help Fibromyalgia & Chronic Fatigue?
-
Researchers have found that D-Ribose can help improve cardiac function and energy levels
Transcription
Synthesis and sources
Ribose as its 5-phosphate ester is typically produced from glucose by the pentose phosphate pathway. In at least some archaea, alternative pathways have been identified.[13]
Ribose can be synthesized chemically, but commercial production relies on fermentation of glucose. Using genetically modified strains of B. subtilis, 90 g/liter of ribose can be produced from 200 g of glucose. The conversion entails the intermediacy of gluconate and ribulose.[14]
Ribose has been detected in meteorites.[15][16]
Structure
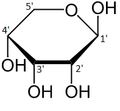
Ribose is an aldopentose (a monosaccharide containing five carbon atoms that, in its open chain form, has an aldehyde functional group at one end). In the conventional numbering scheme for monosaccharides, the carbon atoms are numbered from C1' (in the aldehyde group) to C5'. The deoxyribose derivative found in DNA differs from ribose by having a hydrogen atom in place of the hydroxyl group at C2'. This hydroxyl group performs a function in RNA splicing.
The "d-" in the name d-ribose refers to the stereochemistry of the chiral carbon atom farthest away from the aldehyde group (C4'). In d-ribose, as in all d-sugars, this carbon atom has the same configuration as in d-glyceraldehyde.
-
α-d-Ribopyranose
-
β-d-Ribopyranose
-
α-d-Ribofuranose
-
β-d-Ribofuranose
Relative abundance of forms of ribose in solution: β-d-ribopyranose (59%), α-d-ribopyranose (20%), β-d-ribofuranose (13%), α-d-ribofuranose (7%) and open chain (0.1%).[11]
For ribose residues in nucleosides and nucleotide, the torsion angles for the rotation encompassing the bonds influence the configuration of the respective nucleoside and nucleotide. The secondary structure of a nucleic acid is determined by the rotation of its 7 torsion angles.[17] Having a large amount of torsion angles allows for greater flexibility.
In closed ring riboses, the observed flexibility mentioned above is not observed because the ring cycle imposes a limit on the number of torsion angles possible in the structure.[17] Conformers of closed form riboses differ in regards to how the lone oxygen in the molecule is positioned respective to the nitrogenous base (also known as a nucleobase or just a base) attached to the ribose. If a carbon is facing towards the base, then the ribose is labeled as endo. If a carbon is facing away from the base, then the ribose is labeled as exo. If there is an oxygen molecule attached to the 2' carbon of a closed cycle ribose, then the exo confirmation is more stable because it decreases the interactions of the oxygen with the base.[17] The difference itself is quite small, but when looking at an entire chain of RNA the slight difference amounts to a sizable impact.
- Some pucker configurations of Ribose
-
2' endo
-
2' endo 3' exo
-
3' endo 2' exo
-
3' endo
A ribose molecule is typically represented as a planar molecule on paper. Despite this, it is typically non-planar in nature. Even between hydrogen atoms, the many constituents on a ribose molecule cause steric hindrance and strain between them. To relieve this crowding and ring strain, the ring puckers, i.e. becomes non-planar.[18] This puckering is achieved by displacing an atom from the plane, relieving the strain and yielding a more stable configuration.[17] Puckering, otherwise known as the sugar ring conformation (specifically ribose sugar), can be described by the amplitude of pucker as well as the pseudorotation angle. The pseudo-rotation angle can be described as either "north (N)" or "south (S)" range. While both ranges are found in double helices, the north range is commonly associated with RNA and the A form of DNA. In contrast, the south range is associated with B form DNA. Z-DNA contains sugars in both the north and south ranges.[19] When only a single atom is displaced, it is referred to as an "envelope" pucker. When two atoms are displaced, it is referred to as a "twist" pucker, in reference to the zigzag orientation.[20] In an "endo" pucker, the major displacement of atoms is on the β-face, the same side as the C4'-C5' bond and the base. In an "exo" pucker, the major displacement of atoms is on the α-face, on the opposite side of the ring. The major forms of ribose are the 3'-endo pucker (commonly adopted by RNA and A-form DNA) and 2'-endo pucker (commonly adopted by B-form DNA).[21] These ring puckers are developed from changes in ring torsion angles; there are infinite combinations of angles so therefore, there is an infinite number of transposable pucker conformations, each separated by disparate activation energies.
Functions
ATP is derived from ribose; it contains one ribose, three phosphate groups, and an adenine base. ATP is created during cellular respiration from adenosine diphosphate (ATP with one less phosphate group).
Signaling pathways
Ribose is a building block in secondary signaling molecules such as cyclic adenosine monophosphate (cAMP) which is derived from ATP. One specific case in which cAMP is used is in cAMP-dependent signaling pathways. In cAMP signaling pathways, either a stimulative or inhibitory hormone receptor is activated by a signal molecule. These receptors are linked to a stimulative or inhibitory regulative G-protein. When a stimulative G-protein is activated, adenylyl cyclase catalyzes ATP into cAMP by using Mg2+ or Mn2+. cAMP, a secondary messenger, then goes on to activate protein kinase A, which is an enzyme that regulates cell metabolism. Protein kinase A regulates metabolic enzymes by phosphorylation which causes a change in the cell depending on the original signal molecule. The opposite occurs when an inhibitory G-protein is activated; the G-protein inhibits adenylyl cyclase and ATP is not converted to cAMP.

Metabolism
Ribose is referred to as the "molecular currency" because of its involvement in intracellular energy transfers.[citation needed] For example, nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide phosphate (NADP) all contain the d-ribofuranose moiety. They can each be derived from d-ribose after it is converted to d-ribose 5-phosphate by the enzyme ribokinase.[22][23] NAD, FAD, and NADP act as electron acceptors in biochemical redox reactions in major metabolic pathways including glycolysis, the citric acid cycle, fermentation, and the electron transport chain.
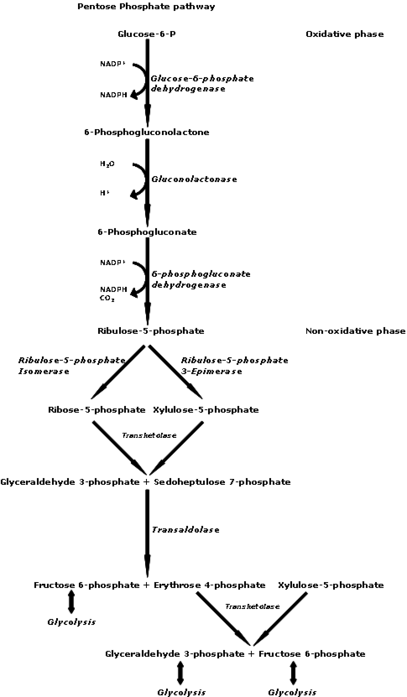
Nucleotide biosynthesis
Nucleotides are synthesized through salvage or de novo synthesis.[24] Nucleotide salvage uses pieces of previously made nucleotides and re-synthesizes them for future use. In de novo, amino acids, carbon dioxide, folate derivatives, and phosphoribosyl pyrophosphate (PRPP) are used to synthesize nucleotides.[24] Both de novo and salvage require PRPP which is synthesized from ATP and ribose 5-phosphate by an enzyme called PRPP synthetase.[24]
Modifications
Modifications in nature
Ribokinase catalyzes the conversion of d-ribose to d-ribose 5-phosphate. Once converted, d-ribose-5-phosphate is available for the manufacturing of the amino acids tryptophan and histidine, or for use in the pentose phosphate pathway. The absorption of d-ribose is 88–100% in the small intestines (up to 200 mg/kg·h).[25]
One important modification occurs at the C2' position of the ribose molecule. By adding an O-alkyl group, the nuclear resistance of the RNA is increased because of additional stabilizing forces. These forces are stabilizing because of the increase of intramolecular hydrogen bonding and an increase in the glycosidic bond stability.[26] The resulting increase of resistance leads to increases in the half-life of siRNA and the potential therapeutic potential in cells and animals.[27] The methylation of ribose at particular sites is correlated with a decrease in immune stimulation.[28]
Synthetic modifications
Along with phosphorylation, ribofuranose molecules can exchange their oxygen with selenium and sulfur to produce similar sugars that only vary at the 4' position. These derivatives are more lipophilic than the original molecule. Increased lipophilicity makes these species more suitable for use in techniques such as PCR, RNA aptamer post-modification, antisense technology, and for phasing X-ray crystallographic data.[27]
Similar to the 2' modifications in nature, a synthetic modification of ribose includes the addition of fluorine at the 2' position. This fluorinated ribose acts similar to the methylated ribose because it is capable of suppressing immune stimulation depending on the location of the ribose in the DNA strand.[26] The big difference between methylation and fluorination, is the latter only occurs through synthetic modifications. The addition of fluorine leads to an increase in the stabilization of the glycosidic bond and an increase of intramolecular hydrogen bonds.[26]
Medical uses
d-ribose has been suggested for use in management of congestive heart failure[29] (as well as other forms of heart disease) and for chronic fatigue syndrome (CFS), also called myalgic encephalomyelitis (ME) in an open-label non-blinded, non-randomized, and non-crossover subjective study.[30]
Supplemental d-ribose can bypass part of the pentose phosphate pathway, an energy-producing pathway, to produce d-ribose-5-phosphate. The enzyme glucose-6-phosphate-dehydrogenase (G-6-PDH) is often in short supply in cells, but more so in diseased tissue, such as in myocardial cells in patients with cardiac disease. The supply of d-ribose in the mitochondria is directly correlated with ATP production; decreased d-ribose supply reduces the amount of ATP being produced. Studies suggest that supplementing d-ribose following tissue ischemia (e.g. myocardial ischemia) increases myocardial ATP production, and therefore mitochondrial function. Essentially, administering supplemental d-ribose bypasses an enzymatic step in the pentose phosphate pathway by providing an alternate source of 5-phospho-d-ribose 1-pyrophosphate for ATP production. Supplemental d-ribose enhances recovery of ATP levels while also reducing cellular injury in humans and other animals. One study suggested that the use of supplemental d-ribose reduces the instance of angina in men with diagnosed coronary artery disease.[31] d-Ribose has been used to treat many pathological conditions, such as chronic fatigue syndrome, fibromyalgia, and myocardial dysfunction. It is also used to reduce symptoms of cramping, pain, stiffness, etc. after exercise and to improve athletic performance[citation needed].
References
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 8205
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-506. ISBN 0-8493-0462-8.
- ^ Fischer, Emil; Piloty, Oscar (1891). "Ueber eine neue Pentonsäure und die zweite inactive Trioxyglutarsäure" [About a new pentonic acid and the second inactive trioxyglutaric acid]. Berichte der deutschen chemischen Gesellschaft (in German). 24 (2): 4214–4225. doi:10.1002/cber.189102402322. Archived from the original on 4 June 2020. Retrieved 12 March 2020.
- ^ Levene, P. A.; Jacobs, W. A. (1909). "Über Inosinsäure" [About inosic acid]. Berichte der deutschen chemischen Gesellschaft (in German). 42 (1): 1198–1203. doi:10.1002/cber.190904201196.
- ^ Levene, P. A.; Jacobs, W. A. (1909). "Über die Pentose in den Nucleinsäuren" [About the pentose in the nucleic acids]. Berichte der deutschen chemischen Gesellschaft (in German). 42 (3): 3247–3251. doi:10.1002/cber.19090420351.
- ^ a b Jeanloz, Roger W.; Fletcher, Hewitt G. (1951). "The Chemistry of Ribose". In Hudson, Claude S.; Cantor, Sidney M. (eds.). Advances in Carbohydrate Chemistry. Vol. 6. Academic Press. pp. 135–174. doi:10.1016/S0096-5332(08)60066-1. ISBN 9780080562650. PMID 14894350. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ Nechamkin, Howard (1958). "Some interesting etymological derivations of chemical terminology". Science Education. 42 (5): 463–474. Bibcode:1958SciEd..42..463N. doi:10.1002/sce.3730420523.
- ^ a b c d Dewick, Paul M. (2013). "Oxygen as a Nucleophile: Hemicetals, Hemiketals, Acetals and Ketals". Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. John Wiley & Sons. pp. 224–234. ISBN 9781118681961. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ Leigh, Jeffery (July–August 2012). "Non-IUPAC Nomenclature Systems". Chemistry International. International Union of Pure and Applied Chemistry. 34 (4). Archived from the original on 5 December 2019. Retrieved 15 December 2019.
- ^ a b c Bhutani, S. P. (2019). "Aldopentoses—The Sugars of Nucleic Acids". Chemistry of Biomolecules (2nd ed.). CRC Press. pp. 63–65. ISBN 9781000650907. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ a b Drew, Kenneth N.; Zajicek, Jaroslav; Bondo, Gail; Bose, Bidisha; Serianni, Anthony S. (February 1998). "13C-labeled aldopentoses: detection and quantitation of cyclic and acyclic forms by heteronuclear 1D and 2D NMR spectroscopy". Carbohydrate Research. 307 (3–4): 199–209. doi:10.1016/S0008-6215(98)00040-8.
- ^ de Wulf, P.; Vandamme, E. J. (1997). "Microbial Synthesis of ᴅ-Ribose: Metabolic Deregulation and Fermentation Process". Advances in Applied Microbiology. 44: 167–214. doi:10.1016/S0065-2164(08)70462-3. ISBN 9780120026449.
- ^ Tumbula, D. L.; Teng, Q.; Bartlett, M. G.; Whitman, W. B. (1997). "Ribose biosynthesis and evidence for an alternative first step in the common aromatic amino acid pathway in Methanococcus maripaludis". Journal of Bacteriology. 179 (19): 6010–6013. doi:10.1128/jb.179.19.6010-6013.1997. PMC 179501. PMID 9324245.
- ^ Wulf, P. De; Vandamme, E. J. (1997). "Production of d -ribose by fermentation". Applied Microbiology and Biotechnology. 48 (2): 141–148. doi:10.1007/s002530051029. hdl:11572/262019. PMID 9299771. S2CID 34340369.
- ^ Steigerwald, Bill; Jones, Nancy; Furukawa, Yoshihiro (18 November 2019). "First Detection of Sugars in Meteorites Gives Clues to Origin of Life". NASA. Archived from the original on 15 January 2021. Retrieved 18 November 2019.
- ^ Furukawa, Yoshihiro; Chikaraishi, Yoshito; Ohkouchi, Naohiko; Ogawa, Nanako O.; Glavin, Daniel P.; Dworkin, Jason P.; Abe, Chiaki; Nakamura, Tomoki (2019). "Extraterrestrial ribose and other sugars in primitive meteorites". Proceedings of the National Academy of Sciences of the United States of America. 116 (49): 24440–24445. Bibcode:2019PNAS..11624440F. doi:10.1073/pnas.1907169116. PMC 6900709. PMID 31740594.
- ^ a b c d Bloomfield, Victor; Crothers, Donald; Tinoco, Ignacio (2000). Nucleic Acids: Structures, Properties, and Functions. University Science Books. pp. 19–25. ISBN 9780935702491.
- ^ Voet, Donald; Voet, Judith (2011). Biochemistry. John Wiley & Sons, Inc. pp. 1152, 1153. ISBN 978-0470570951.
- ^ Foloppe, Nicolas; MacKerell, Alexander D. (August 1998). "Conformational Properties of the Deoxyribose and Ribose Moieties of Nucleic Acids: A Quantum Mechanical Study". The Journal of Physical Chemistry B. 102 (34): 6669–6678. doi:10.1021/jp9818683. ISSN 1520-6106.
- ^ "Nucleic acid architecture". fbio.uh.cu. Archived from the original on 17 May 2018. Retrieved 8 October 2019.
- ^ Neidle, Stephen (2008). "The Building-Blocks of DNA and RNA". In Neidle, Stephen (ed.). Principles of Nucleic Acid Structure. Academic Press. pp. 20–37. doi:10.1016/B978-012369507-9.50003-0. ISBN 9780123695079.
- ^ Bork, Peer; Sander, Chris; Valencia, Alfonso (1993). "Convergent evolution of similar enzymatic function on different protein folds: The hexokinase, ribokinase, and galactokinase families of sugar kinases". Protein Science. 2 (1): 31–40. doi:10.1002/pro.5560020104. PMC 2142297. PMID 8382990.
- ^ Park, Jae; Gupta, Radhey S. (2008). "Adenosine kinase and ribokinase – the RK family of proteins". Cellular and Molecular Life Sciences. 65 (18): 2875–2896. doi:10.1007/s00018-008-8123-1. PMID 18560757. S2CID 11439854.
- ^ a b c Puigserver, Pere (2018). "Signaling Transduction and Metabolomics". In Hoffman, Ronald; Benz, Edward J.; Silberstein, Leslie E.; Heslop, Helen E. (eds.). Hematology (7th ed.). Elsevier. pp. 68–78. doi:10.1016/B978-0-323-35762-3.00007-X. ISBN 9780323357623.
- ^ "Herbal Remedies, Supplements A-Z Index". PDRHealth.com. PDR, LLC. Archived from the original on 11 October 2008.
- ^ a b c Hamlow, Lucas; He, Chenchen; Fan, Lin; Wu, Ranran; Yang, Bo; Rodgers, M. T.; Berden, Giel; Oomens, J. (June 2015). Structual [sic] Effects of Cytidine 2'-Ribose Modifications as Determined by Irmpd Action Spectroscopy. 70th International Symposium on Molecular Spectroscopy. University of Illinois Urbana-Champaign. Bibcode:2015isms.confEMI13H. doi:10.15278/isms.2015.MI13.
- ^ a b Evich, Marina; Spring-Connell, Alexander M.; Germann, Markus W. (27 January 2017). "Impact of modified ribose sugars on nucleic acid conformation and function". Heterocyclic Communications. 23 (3): 155–165. doi:10.1515/hc-2017-0056. ISSN 2191-0197. S2CID 91052034.
- ^ Peacock, Hayden; Fucini, Raymond V.; Jayalath, Prasanna; Ibarra-Soza, José M.; Haringsma, Henry J.; Flanagan, W. Michael; Willingham, Aarron; Beal, Peter A. (2011). "Nucleobase and Ribose Modifications Control Immunostimulation by a MicroRNA-122-mimetic RNA". Journal of the American Chemical Society. 133 (24): 9200–9203. doi:10.1021/ja202492e. PMC 3116021. PMID 21612237.
- ^ Omran, Heyder; McCarter, Dean; St Cyr, John; Lüderitz, Berndt (2004). "ᴅ-Ribose aids congestive heart failure patients". Experimental & Clinical Cardiology. Summer (9(2)): 117–118. PMC 2716264. PMID 19641697.
- ^ Teitelbaum, Jacob E.; Johnson, Clarence; St Cyr, John (26 November 2006). "The use of ᴅ-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study". The Journal of Alternative and Complementary Medicine. 12 (9): 857–862. CiteSeerX 10.1.1.582.4800. doi:10.1089/acm.2006.12.857. PMID 17109576.
- ^ "Ribose". wa.kaiserpermanente.org. Archived from the original on 3 March 2021. Retrieved 7 October 2019.