| |
| Names | |
|---|---|
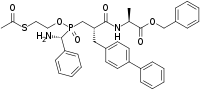
| IUPAC name
Benzyl N-{(2S)-2-[([2-(acetylsulfanyl)ethoxy]{[(R)-amino(phenyl)methyl]phosphonoyl})methyl]-3-([1,1′-biphenyl]-4-yl)propanoyl}-L-alaninate
| |
| Systematic IUPAC name
Benzyl (9S,12S)-7-[(R)-amino(phenyl)methyl]-9-[([1,1′-biphenyl]-4-yl)methyl]-12-methyl-2,7,10-trioxo-6-oxa-3-thia-11-aza-7λ5-phosphatridecan-13-oate | |
| Other names
RB3007, LS-15768
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C37H41N2O6PS | |
| Molar mass | 672.78 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
RB-3007 is an orally active analogue of RB-101. It acts as an enkephalinase inhibitor, which is used in scientific research.[1][2]
See also
- RB-101 - enkephalinase inhibitor that produces analgesia without respiratory depression
- D/DL-Phenylalanine - D-phenylalanine blockage of enkephalin degradation may be responsible for the reputed analgesic effect of DL-Phenylalanine
- Racecadotril - an antidiarrheal drug which acts as a peripheral enkephalinase inhibitor
- Ecadotril - a neutral endopeptidase inhibitor
References
- ^ Thanawala, V.; Kadam, V.; Ghosh, R. (1 October 2008). "Enkephalinase Inhibitors: Potential Agents for the Management of Pain". Current Drug Targets. 9 (10): 887–894. doi:10.2174/138945008785909356. PMID 18855623.
- ^ Noble, Florence; Roques, Bernard P (17 January 2007). "Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs". Expert Opinion on Therapeutic Targets. 11 (2): 145–159. doi:10.1517/14728222.11.2.145. PMID 17227231. S2CID 24437682.
