| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2λ6-Oxathiolane-2,2-dione | |
| Other names
γ-Propane sultone; 1,2-Oxathiolane, 2,2-dioxide; 3-Hydroxyl-1-propane sulfonic acid sulfone; 1-Propane sulfonic acid-3-hydroxyl-γ-sultone; Oxathiolane 2,2-dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.017 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6O3S | |
| Molar mass | 122.14 g·mol−1 |
| Appearance | White crystalline solid; colorless liquid above 31 °C |
| Density | 1.392 g/cm3 at 40 °C |
| Melting point | 31 °C (88 °F; 304 K) |
| Boiling point | 112 °C (234 °F; 385 K) at 1.4 mm Hg |
| 10% (20°C)[1] | |
| Hazards | |
| Flash point | 158 °C (316 °F; 431 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [N.D.][1] |
| Safety data sheet (SDS) | NIH.gov |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones.[2][3] It is a readily melting colorless solid.
YouTube Encyclopedic
-
1/2Views:1 94163 844
-
Limn0.8Fe0.2Po4: An Advanced Cathode Material For Rechargeable Lithium Batteries
-
Professor Jeff Dahn | WIN Seminar Series
Transcription
Synthesis
It may be prepared by the acid catalyzed reaction of allyl alcohol and sodium bisulfite.
Reactions
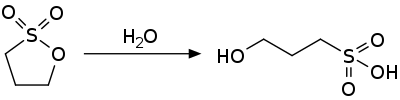
1,3-propane sultone is an activated ester and is susceptible to nucleophilic attack. It hydrolyzes to the 3-hydroxypropylsulfonic acid.
It has been used in the synthesis of specialist surfactants, such as CHAPS detergent.[4]
Safety
Typical of activated esters, 1,3-propane sultone is an alkylating agent. 1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic.[5][6]
See also
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0525". National Institute for Occupational Safety and Health (NIOSH).
- ^ R. J. Cremlyn (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0-471-95512-4.
- ^ Morimoto, Yoshiki; Kurihara, Hajime; Kinoshita, Takamasa (2000). "Can α-sultone exist as a chemical species? First experimental implication for intermediacy of α-sultone" (PDF). Chemical Communications (3): 189–190. doi:10.1039/A909094K.
- ^ Hjelmeland, LM (November 1980). "A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis". Proceedings of the National Academy of Sciences of the United States of America. 77 (11): 6368–70. Bibcode:1980PNAS...77.6368H. doi:10.1073/pnas.77.11.6368. PMC 350285. PMID 6935651.
- ^ "Scorecard Chemical Profile for Propane Sultone". Archived from the original on 2008-09-23. Retrieved 2008-11-17.
- ^ "NIOSH Pocket Guide to Chemical Hazards". Retrieved 2013-11-13.