Names
IUPAC name
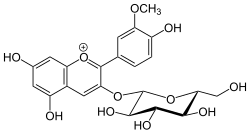
3-(β-D -Glucopyranosyloxy)-4′,5,7-trihydroxy-3′-methoxyflavylium
Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-{[(2S ,3R ,4S ,5S ,6R )-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4 -benzopyran-1-ylium
Other names
Peonidin-3-glucosideO -glucoside
Identifiers
ChEBI
ChemSpider
KEGG
InChI=1S/C22H22O11/c1-30-15-4-9(2-3-12(15)25)21-16(7-11-13(26)5-10(24)6-14(11)31-21)32-22-20(29)19(28)18(27)17(8-23)33-22/h2-7,17-20,22-23,27-29H,8H2,1H3,(H2-,24,25,26)/p+1/t17-,18-,19+,20-,22-/m1/s1
Key: ZZWPMFROUHHAKY-OUUKCGNVSA-O
COC1=C(C=CC(=C1)C2=C(C=C3C(=CC(=CC3=[O+]2)O)O)OC4C(C(C(C(O4)CO)O)O)O)O
Properties
C22 23 + 11 22 H23 O11 Cl (chloride)
Molar mass
463.41 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Peonidin-3-O -glucoside is anthocyanin . It is found in fruits and berries, in red Vitis vinifera red wine ,[1] red onions and in purple corn .[2]
See also References
3-Hydroxyanthocyanidins 3-Deoxyanthocyanidins O -Methylated anthocyanidinsAnthocyanins Glucosides:
Diglucosides:
Cyanin (Cyanidin 3,5-O -diglucoside )
Delphin (Delphinidin 3,5-<i>O</i>-diglucoside)
Malvin (Malvidin 3,5-diglucoside)Pelargonin (Pelargonidin 3,5-O -diglucoside)Peonin (Peonidin 3,5-<i>O</i>-diglucoside)
Petunin (Petunidin 3,5-<i>O</i>-diglucoside) Others glycosides:
Antirrhinin (Cyanidin 3-O -rutinoside)Ideain (Cyanidin 3-O -galactoside)Delphinidin 3-<i>O</i>-rhamnoside
Petunidin 3-<i>O</i>-arabinoside
Petunidin 3-<i>O</i>-galactoside
Petunidin 3-<i>O</i>-rhamnoside
Petunidin 3-<i>O</i>-rutinoside
Primulin (Malvidin 3-O -galactoside)Pulchellidin 3-rhamnoside
Tulipanin (Delphinidin 3-O -rutinoside)Acylated anthocyanins
Acetylated anthocyanins
Cyanidin 3-<i>O</i>-(6-acetyl)glucoside
Delphinidin 3-<i>O</i>-(6-acetyl)glucoside
Malvidin 3-<i>O</i>-(6-acetyl)glucoside
Petunidin 3-<i>O</i>-(6-acetyl)galactoside
Petunidin 3-<i>O</i>-(6-acetyl)glucoside
Peonidin 3-<i>O</i>-(6-acetyl)glucoside
Coumaroylated anthocyaninscis - and trans -) Caffeoylated anthocyanins
Malvidin 3-<i>O</i>-(6-<i>p</i>-caffeoyl)glucoside
Peonidin 3-<i>O</i>-(6-<i>p</i>-caffeoyl)glucoside Malonylated anthocyanins
Malonylmalvin (malvidin 3-(6″-malonylglucoside)-5-glucoside) Acylated anthocyanin diglycosides
Cyanidin 3-<i>O</i>-(di-<i>p</i>-coumarylglucoside)-5-glucoside
Gentiodelphin (delphinidin 3-''O''-glucosyl-5-''O''-(6-''O''-caffeoyl-glucosyl)-3′-''O''-(6-''O''-caffeoyl-glucoside))
Nasunin (Delphinidin 3-(<i>p</i>-coumaroylrutinoside)-5-glucoside)Petanin (petunidin 3-[6-O -(4-O -(E )-p -coumaroyl-O -α-l-rhamnopyranosyl)-β-D -glucopyranoside]-5-O -β-D -glucopyranoside)
Violdelphin (Delphinidin 3-rutinoside-7-O -(6-O -(4-(6-O -(4-hydroxybenzoyl)-β-D -glucosyl)oxybenzoyl)-β-D -glucoside)
Flavanol-anthocyanin adducts
Malvidin glucoside-ethyl-catechin Catechin(4α→8)pelargonidin 3-<i>O</i>-β-glucopyranoside
Epicatechin(4α→8)pelargonidin 3-<i>O</i>-β-glucopyranoside
Afzelechin(4α→8)pelargonidin 3-<i>O</i>-β-glucopyranoside
Epiafzelechin(4α→8)pelargonidin 3-<i>O</i>-β-glucopyranoside Miscellaneous
This page was last edited on 6 May 2023, at 13:21