 | |
| Clinical data | |
|---|---|
| Other names | GYKI-13504; EGIS-5650 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
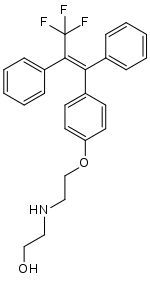
| Formula | C25H24F3NO2 |
| Molar mass | 427.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Panomifene (INN; developmental codes GYKI 13504 and EGIS 5650) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group related to tamoxifen that was under development as an antineoplastic agent by Egis Pharmaceuticals and IVAX Drug Research Institute in the 1990s for the treatment of breast cancer, but it was never marketed.[1][2][3][4][5][6] It reached phase II clinical trials before development was terminated.[2] The drug was described in 1981.[1]
References
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 930–. ISBN 978-1-4757-2085-3.
- ^ a b "Panomifene". AdisInsight. Springer Nature Switzerland AG.
- ^ von Angerer E (6 December 2012). "Antiestrogens and Partial Agonists". In Oettel M, Schillinger E (eds.). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 60–. ISBN 978-3-642-58616-3.
- ^ Sitruk-Ware R (6 December 2012). "Pharmacological Different Administration Routes - Oral vs Transdermal". In Oettel M, Schillinger E (eds.). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 292–. ISBN 978-3-642-60107-1.
- ^ Koike T, Akita M (18 October 2014). "Visible-Light-Induced Redox Reactions by Ruthenium Photoredox Catalyst". In Dixneuf PH, Bruneau C (eds.). Ruthenium in Catalysis. Springer. pp. 385–. ISBN 978-3-319-08482-4.
- ^ Borvendég J, Hermann I, Csuka O (1996). "Antiestrogens, antiandrogens". Acta Physiologica Hungarica. 84 (4): 405–406. PMID 9328614.
