| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6OS | |
| Molar mass | 90.14 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.1779 g/cm³ |
| Boiling point | 127–129 °C (261–264 °F; 400–402 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
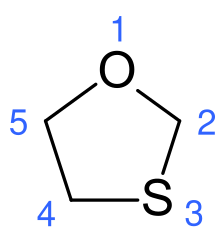
1,3-Oxathiolane is an organosulfur compound with the formula (CH2)3OS. It is a saturated five-membered heterocycle with non-adjacent S and O atoms. It is the parent of numerous derivatives. The parent compound is of little practical value, but there is some biotechnological interest in derivatives where one or more H atoms are replaced by other substituents.[1]
Preparation and occurrence

Apricitabine, a drug candidate containing a 1,3-oxathiane, is a nucleoside reverse transcriptase inhibitor.
The compound is prepared by condensation of mercaptoethanol with formaldehyde, as typical for synthesis of thioacetals.[2]
2-Methyl-4-propyl-1,3-oxathiane is a component of the flavor of passion fruit and other fruits.[3]
1,2-Oxathiolane
In contrast to the well-developed area of 1,3-oxathiolanes, 1,2-oxathiolane and its derivatives are not prevalent in the literature. The parent compound, which is derived from 3-mercaptopropanol, has been detected in solution [4] A bulky derivative has been characterized by X-ray crystallography.[5]
References
- ^ "1,3-Oxathiolane".
- ^ Djerassi, Carl; Gorman, Marvin (1953). "Studies in Organic Sulfur Compounds. VI. Cyclic Ethylene and Trimethylene Hemithioketals". Journal of the American Chemical Society. 75 (15): 3704–3708. doi:10.1021/ja01111a029.
- ^ Porto-Figueira, Priscilla; Freitas, Ana; Cruz, Catarina J.; Figueira, José; Câmara, José S. (2015). "Profiling of passion fruit volatiles: An effective tool to discriminate between species and varieties". Food Research International. 77: 408–418. doi:10.1016/j.foodres.2015.09.007.
- ^ Davis, Anthony P.; Whitham, Gordon H. (1981). "1,2-Oxathiolan, a simple sultene". Journal of the Chemical Society, Chemical Communications (15): 741. doi:10.1039/C39810000741.
- ^ Baldwin, Jack E.; Herchen, Stephen R.; Schulz, Guenter; Falshaw, Christopher P.; King, Trevor J. (1980). "Rearrangement of penicillin sulfoxides in base. Penicillin-derived sulfines". Journal of the American Chemical Society. 102 (26): 7815–7816. doi:10.1021/ja00546a047.

