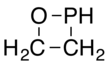| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
1,2-Oxaphosphetane
1,3-Oxaphosphetane | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C2H5OP | |||
| Molar mass | 76.035 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

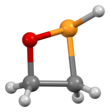
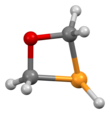
An oxaphosphetane is a molecule containing a four-membered ring with one phosphorus, one oxygen and two carbon atoms. In a 1,2-oxaphosphetane phosphorus is bonded directly to oxygen, whereas a 1,3-oxaphosphetane has the phosphorus and oxygen atoms at opposite corners.
1,2-Oxaphosphetanes are rarely isolated but are important intermediates in the Wittig reaction and related reactions such as the Seyferth–Gilbert homologation and the Horner–Wadsworth–Emmons reaction.[2] Edwin Vedejs's NMR studies first revealed the importance of oxaphosphetanes in the mechanism of the Wittig reaction in the 1970s.[3][4]
In 2005 the first isolation of 1,2-Oxaphosphetanes (typical Wittig intermediates) was reported.[5] One of the compounds was characterized by X-ray crystallography and NMR. Although relatively stable, thermal decomposition of these oxaphosphetanes gave a phosphonium salt, which slowly dissociated to the Wittig reaction starting materials, the carbonyl and olefin compounds.
References
- ^ M. Hamaguchi; Y. Iyamaa; E. Mochizukia; T. Oshima (2005). "First isolation and characterization of 1,2-oxaphosphetanes with three phenyl groups at the phosphorus atom in typical Wittig reaction using cyclopropylidenetriphenylphosphorane". Tetrahedron Letters. 46 (51): 8949–8952. doi:10.1016/j.tetlet.2005.10.086.
- ^ Byrne, Peter A.; Gilheany, Declan G. (2013). "The modern interpretation of the Wittig reaction mechanism". Chemical Society Reviews. 42 (16): 6670–6696. doi:10.1039/C3CS60105F. hdl:10197/4939. PMID 23673458.
- ^ Vedejs E (30 July 2004). "Studies in Heteroelement-Based Synthesis". The Journal of Organic Chemistry. 69 (16): 5159–5167. doi:10.1021/jo049360l. PMID 15287757.
- ^ "Memorial Resolution of the Faculty of the University of Wisconsin-Madison" (PDF). University of Wisconsin-Madison. Archived from the original (PDF) on 9 May 2020. Retrieved 9 May 2020.
- ^ M. Hamaguchi; Y. Iyamaa; E. Mochizukia; T. Oshima (2005). "First isolation and characterization of 1,2-oxaphosphetanes with three phenyl groups at the phosphorus atom in typical Wittig reaction using cyclopropylidenetriphenylphosphorane". Tetrahedron Letters. 46 (51): 8949–8952. doi:10.1016/j.tetlet.2005.10.086.