
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Pentylbenzene-1,3-diol | |
| Other names
5-Pentyl-1,3-benzenediol; 5-Pentylresorcinol; 5-n-Amylresorcinol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.190 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H16O2 | |
| Molar mass | 180.247 g·mol−1 |
| Melting point | 40 to 41 °C (104 to 106 °F; 313 to 314 K) (49 to 49.5 °C) |
| Boiling point | 162 to 164 °C (324 to 327 °F; 435 to 437 K) at 5 mmHg; 192-195 °C at 11 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Olivetol, also known as 5-pentylresorcinol or 5-pentyl-1,3-benzenediol, is an organic compound found in certain species of lichen; it is also a precursor in various syntheses of tetrahydrocannabinol.
YouTube Encyclopedic
-
1/3Views:104 04215 53652 668
-
Lec 5 | MIT 5.301 Chemistry Laboratory Techniques, IAP 2004
-
How to Perform an Extraction - Separatory Funnel
-
Isolation of Plant Pigments by Column Chromatography - Amrita University
Transcription
Occurrence
Olivetol is a naturally occurring organic compound. It is found in certain species of lichens and can be readily extracted.[1]
Olivetol is also produced by a number of insects, either as a pheromone, repellent, or antiseptic.[2][3]
The cannabis plant internally produces the related substance olivetolic acid (OLA), which may be involved in the biosynthesis of tetrahydrocannabinol (THC).[4][5]
Synthesis of THC analogs
Olivetol is used in various methods to produce synthetic analogs of THC.[6][full citation needed] One such method is a condensation reaction of olivetol and pulegone.[citation needed] In PiHKAL, Alexander Shulgin reports a cruder method of producing the same product by bringing to reaction olivetol and the essential oil of orange in the presence of phosphoryl chloride.[7]
A method for the synthesis of THC itself consists of the condensation reaction between olivetol and Δ2-carene oxide.[8]
Legality
The production, possession, and/or distribution of olivetol is not outlawed by any country; however, in the United States, it is a DEA watched precursor.[9][unreliable source?]
Biosynthesis
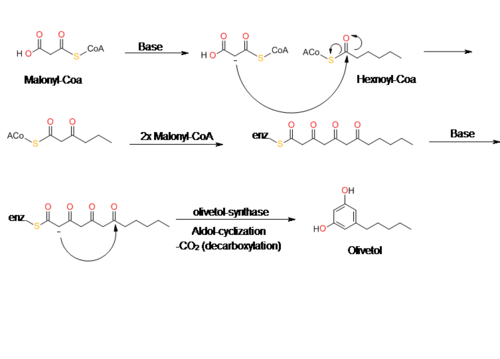
Olivetol is biosynthesized by a polyketide synthase (PKS)-type reaction from hexanoyl-CoA and three molecules of malonyl-CoA by an aldol condensation of a tetraketide intermediate. In 2009, Taura et al. was able to clone a type III PKS named olivetol synthase (OLS) from Cannabis sativa.[10] This PKS is a homodimeric protein that consists of a 385 amino acid polypeptide with a molecular mass of 42,585 Da that has high sequence similarity (60-70%) identity to plant PKS's.[10]
The data from Taura's study of OLS's enzyme kinetics show that OLS catalyzes a decarboxylative-aldol condensation to produce olivetol. This is similar to stilbene synthase's (STS) mechanism for converting p-coumaroyl-CoA and malonyl-CoA to resveratrol. Although olivetol is the decarboxylated form of OLA, it is highly unlikely that OLS produces olivetol from OLA.[10][11] Crude enzyme extracts prepared from flowers and leaves did not synthesize olivetolic acid, but only yielded olivetol.[10] The exact mechanism of olivetol biosynthesis is as yet unsure, but it is possible that an OLA-forming metabolic complex forms along with OLS.[10] In addition, it also appears that OLS only specifically accepts starter CoA esters with C4 to C8 aliphatic side chains such as hexanoyl-CoA.[10][12]
References
- ^ Oettl, Sarah K.; Gerstmeier, Jana; Khan, Shafaat Y.; Wiechmann, Katja; Bauer, Julia; Atanasov, Atanas G.; Malainer, Clemens; Awad, Ezzat M.; Uhrin, Pavel; Heiss, Elke H.; Waltenberger, Birgit; Remias, Daniel; Breuss, Johannes M.; Boustie, Joel; Dirsch, Verena M.; Stuppner, Hermann; Werz, Oliver; Rollinger, Judith M. (2013). Johnson, Christopher James (ed.). "Imbricaric Acid and Perlatolic Acid: Multi-Targeting Anti-Inflammatory Depsides from Cetrelia monachorum". PLOS ONE. 8 (10): e76929. Bibcode:2013PLoSO...876929O. doi:10.1371/journal.pone.0076929. PMC 3793931. PMID 24130812.
- ^ Attygalle et al. (1989). Journal of Chemical Ecology. (15) 1: 317-28 ISSN 0098-0331/89/0100-0317506.00/0
- ^ The Pherobase (Database of pheromones and semiochemicals). 5-Pentylresorcinol. Retrieved 18 January 2014
- ^ Hassuni, I; Razxouk, H (2005). "Olivetol: Constituent of lichen Evernia prunastri Ach. or "oakmoss"". Physical and Chemical News. 26: 98–103. Archived from the original on 3 September 2019. Retrieved 18 October 2013.
- ^ Stojanovic, Igor; Radulovic, Niko; Mitrovic, Tatjana; Stamenkovic, Slavisa; Stojanovic, Gordana (2011). "Volatile constituents of selected Parmeliaceae lichens". Journal of the Serbian Chemical Society. 76 (7): 987–94. doi:10.2298/JSC101004087S.
- ^ Adams, Roger University of Illinois
- ^ Shulgin, Alexander T (1991) PiHKAL: 26
- ^ US 3734930, Razdan, Raj Kumar & Handrick, Richard G., "DIRECT SYNTHESIS OF (-)-TRANS-Δ TETRAHYDROCANNABINOL FROM OLIVETOL AND (+)-TRANS-Δ-CARENE OXIDE"
- ^ "Erowid Psychoactive Vaults : DEA Watched Chemicals".
- ^ a b c d e f Taura, Futoshi; Tanaka, Shinji; Taguchi, Chiho; Fukamizu, Tomohide; Tanaka, Hiroyuki; Shoyama, Yukihiro; Morimoto, Satoshi (2009). "Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway". FEBS Letters. 583 (12): 2061–6. doi:10.1016/j.febslet.2009.05.024. PMID 19454282. S2CID 13746503.
- ^ Raharjo, Tri J; Chang, Wen-Te; Choi, Young Hae; Peltenburg-Looman, Anja M.G; Verpoorte, Robert (2004). "Olivetol as product of a polyketide synthase in Cannabis sativa L". Plant Science. 166 (2): 381–5. doi:10.1016/j.plantsci.2003.09.027.
- ^ Raharjo, Tri J.; Chang, Wen-Te; Verberne, Marianne C.; Peltenburg-Looman, Anja M.G.; Linthorst, Huub J.M.; Verpoorte, Robert (2004). "Cloning and over-expression of a cDNA encoding a polyketide synthase from Cannabis sativa". Plant Physiology and Biochemistry. 42 (4): 291–7. doi:10.1016/j.plaphy.2004.02.011. PMID 15120113.
