 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.320 |
| Chemical and physical data | |
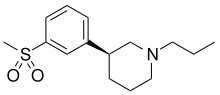
| Formula | C15H23NO2S |
| Molar mass | 281.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
OSU-6162 (PNU-96391) is a compound which acts as a partial agonist at both dopamine D2 receptors and 5-HT2A receptors. It acts as a dopamine stabilizer in a similar manner to the closely related drug pridopidine, and has antipsychotic, anti-addictive and anti-Parkinsonian effects in animal studies.[1][2][3][4][5][6][7][8][9][10][11][12][13][14] Both enantiomers show similar activity but with different ratios of effects, with the (S) enantiomer (–)-OSU-6162 that is more commonly used in research, having higher binding affinity to D2 but is a weaker partial agonist at 5-HT2A, while the (R) enantiomer (+)-OSU-6162 has higher efficacy at 5-HT2A but lower D2 affinity.[15][16]
YouTube Encyclopedic
-
1/3Views:1 9556 5937 933
-
History of Neuroscience: Arvid Carlsson
-
Quanser 2DOF helicopter tracking joystick under LQR+integrator+observer control
-
ATRIAS: An Agile and Efficient Bipedal Robot
Transcription
See also
References
- ^ Ekesbo A, Andrén PE, Gunne LM, Tedroff J (July 1997). "(-)-OSU 6162 inhibits levodopa-induced dyskinesias in a monkey model of Parkinson's disease". NeuroReport. 8 (11): 2567–70. doi:10.1097/00001756-199707280-00029. PMID 9261828. S2CID 29806568.
- ^ Tedroff J, Torstenson R, Hartvig P, Sonesson C, Waters N, Carlsson A, et al. (April 1998). "Effects of the substituted (S)-3-phenylpiperidine (-)-OSU6162 on PET measurements in subhuman primates: evidence for tone-dependent normalization of striatal dopaminergic activity". Synapse. 28 (4): 280–7. doi:10.1002/(SICI)1098-2396(199804)28:4<280::AID-SYN3>3.0.CO;2-5. PMID 9517836. S2CID 2090820.
- ^ Tedroff J, Ekesbo A, Sonesson C, Waters N, Carlsson A (October 1999). "Long-lasting improvement following (-)-OSU6162 in a patient with Huntington's disease". Neurology. 53 (7): 1605–6. doi:10.1212/wnl.53.7.1605. PMID 10534281.
- ^ Nichols NF, Cimini MG, Haas JV, Staton BA, Tedroff J, Svensson KA (October 2002). "PNU-96391A (OSU6162) antagonizes the development of behavioral sensitization induced by dopamine agonists in a rat model for Parkinson's disease". Neuropharmacology. 43 (5): 817–24. doi:10.1016/s0028-3908(02)00144-2. PMID 12384167. S2CID 22101873.
- ^ Tamminga CA, Carlsson A (April 2002). "Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis". Current Drug Targets. CNS and Neurological Disorders. 1 (2): 141–7. doi:10.2174/1568007024606195. PMID 12769623.
- ^ Brandt-Christensen M, Andersen MB, Fink-Jensen A, Werge T, Gerlach J (January 2006). "The substituted (S)-3-phenylpiperidine (-)-OSU6162 reduces apomorphine- and amphetamine-induced behaviour in Cebus apella monkeys". Journal of Neural Transmission. 113 (1): 11–9. doi:10.1007/s00702-005-0297-1. PMID 15795789. S2CID 21099503.
- ^ Rung JP, Carlsson A, Markinhuhta KR, Carlsson ML (June 2005). "The dopaminergic stabilizers (-)-OSU6162 and ACR16 reverse (+)-MK-801-induced social withdrawal in rats". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 29 (5): 833–9. doi:10.1016/j.pnpbp.2005.03.003. PMID 15913873. S2CID 20868201.
- ^ Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, Kapur S (August 2006). "The dopamine stabilizers (S)-(-)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(-)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat". The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 810–8. doi:10.1124/jpet.106.102905. PMID 16648369. S2CID 9385308.
- ^ Seeman P, Guan HC (February 2007). "Dopamine partial agonist action of (-)OSU6162 is consistent with dopamine hyperactivity in psychosis". European Journal of Pharmacology. 557 (2–3): 151–3. doi:10.1016/j.ejphar.2006.11.016. PMID 17157291.
- ^ Lahti RA, Tamminga CA, Carlsson A (September 2007). "Stimulating and inhibitory effects of the dopamine "stabilizer" (-)-OSU6162 on dopamine D2 receptor function in vitro". Journal of Neural Transmission. 114 (9): 1143–6. doi:10.1007/s00702-007-0784-7. PMID 17612788. S2CID 23496944.
- ^ Rung JP, Rung E, Helgeson L, Johansson AM, Svensson K, Carlsson A, Carlsson ML (June 2008). "Effects of (-)-OSU6162 and ACR16 on motor activity in rats, indicating a unique mechanism of dopaminergic stabilization". Journal of Neural Transmission. 115 (6): 899–908. doi:10.1007/s00702-008-0038-3. PMID 18351286. S2CID 20687327.
- ^ Benaliouad F, Kapur S, Natesan S, Rompré PP (June 2009). "Effects of the dopamine stabilizer, OSU-6162, on brain stimulation reward and on quinpirole-induced changes in reward and locomotion". European Neuropsychopharmacology. 19 (6): 416–30. doi:10.1016/j.euroneuro.2009.01.014. PMID 19269794. S2CID 19419177.
- ^ Dyhring T, Nielsen EØ, Sonesson C, Pettersson F, Karlsson J, Svensson P, et al. (February 2010). "The dopaminergic stabilizers pridopidine (ACR16) and (-)-OSU6162 display dopamine D(2) receptor antagonism and fast receptor dissociation properties". European Journal of Pharmacology. 628 (1–3): 19–26. doi:10.1016/j.ejphar.2009.11.025. PMID 19919834.
- ^ Kara E, Lin H, Svensson K, Johansson AM, Strange PG (November 2010). "Analysis of the actions of the novel dopamine receptor-directed compounds (S)-OSU6162 and ACR16 at the D2 dopamine receptor". British Journal of Pharmacology. 161 (6): 1343–50. doi:10.1111/j.1476-5381.2010.01010.x. PMC 3000658. PMID 20804495.
- ^ Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, et al. (November 2011). "I. In vivo evidence for partial agonist effects of (-)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors". Journal of Neural Transmission. 118 (11): 1511–22. doi:10.1007/s00702-011-0704-8. PMID 21874578. S2CID 43580367.
- ^ Burstein ES, Carlsson ML, Owens M, Ma JN, Schiffer HH, Carlsson A, Hacksell U (November 2011). "II. In vitro evidence that (-)-OSU6162 and (+)-OSU6162 produce their behavioral effects through 5-HT2A serotonin and D2 dopamine receptors". Journal of Neural Transmission. 118 (11): 1523–33. doi:10.1007/s00702-011-0701-y. PMID 21866391. S2CID 763630.
