Nitrosation is a process of converting organic compounds into nitroso derivatives, i.e., compounds containing the R-NO functionality.
YouTube Encyclopedic
-
1/3Views:195 312281 12016 551
-
Amine naming introduction | Amines | Organic chemistry | Khan Academy
-
Electrophilic aromatic substitution | Aromatic Compounds | Organic chemistry | Khan Academy
-
NitroBenzene to Aniline - Nitro to Amine Reduction Mechanism - Proposed Mechanism
Transcription
In this video we're going to talk a little bit about amines. And these are just organic compounds where you have a nitrogen bonded to groups that contain carbon. So if I were to just draw some amines right here, you could have something like this where you have a nitrogen bonded to two hydrogens and then maybe some type of carbon chain. Maybe it's just one carbon right here. So maybe you have a carbon, which is also bonded to three hydrogens like this. In this case you would have a primary amine. If you have the nitrogen bonded to two carbons. So if you have something like this, a nitrogen bonded to two carbons. So it has one carbon chain right there. And I'm just drawing methyl groups here. The chain could keep on going. Maybe you have two like that. And then you have a hydrogen right there. This would be a secondary amine. And then finally you could imagine, if you had it bonded to three, it would be a tertiary amine. And this is just to get you introduced to the terminology. CH3, just like that. Now like all of the other new groups or new types of compounds that we've explored, what I want to do is just introduce you to the naming of it. Because one, that lets you recognize them when you hear their names. But it also, I think on some level, familiarizes you with their structure. So let's do a couple of naming examples. I drew these ahead of time. And in general, just to remember, amines are a pretty high-priority group. Out of all the things that we've learned so far, the only thing that is a higher priority is actually the alcohols. Or actually the thiols, which I don't even remember if I did a video on it. But thiols are just like alcohols, but instead of an oxygen you have a sulfur. So this comes right after those. So let's think about this one right here. So we always want to look for our longest carbon chain. And our longest carbon chain is right here. One, two, three, four, five, six, seven carbons. You want to start numbering it closer to the functional group. One, two, three, four, five, six, and seven. And then the functional group is on the two carbon right there. So first of all, seven carbons. We would use the prefix hept. So it would be heptan. Let me write that down. So it'd be heptan. And since our functional group is an amine in this situation, it has a higher priority than the fact this is an alkane. So this will actually define the suffix. So we would then say well then on the two carbon-- let me do this in new color-- on the two carbon right over here we have the amine group. So it's heptan-2-amine. And we're done. We know that we have an amine group on the two carbon. Now let's do this one. This one's a little bit more hairy. So first of all, we always want to figure out the longest carbon chain. And it looks like the ring is going to be the longest carbon chain. We have one, two, three, four, five, six carbons. So this thing right here is the longest chain. We only have one, two carbons right there. One carbon and one carbon, right over there. So our root is going to be cyclohexane. I want to make sure I have enough space. Cyclohexane. And then once again it's an amine, so this is going to take higher priority. So we're not just going to put an e at the end and call this cyclohexane. This takes higher priority. So it will actually define the suffix. And another thing to think about is you could say, OK this is going to be the one, this is going to be the one carbon. And so you could call this cyclohexan-1-amine. But in general, if this is defining it, you always assume that you're going to start numbering right there at number one. So you could just call this cyclohexanamine. If it's written like this you assume that the one carbon is where the amine group is attached. Now what else do we have on this thing? We took care of the amine. We took care of the cyclo. Well we have this ether right here. And this ether has one, two carbons. If it was just a two carbon chain, it would be ethane. But since we have it's an ether, it's bonded to this oxygen, we call this ethoxy. So that right there is ethoxy. And we're going to have to think about how we're going to number this. So I'll leave that alone. And what are these over here? Well this is a methyl group. That's a CH3 implicitly. You don't see it drawn. There's a carbon there. And if carbon is neutral, it has to have four bonds. And if you only draw one of them, the other three are assumed to be the hydrogen. So this is a methyl group. And then this is also a methyl group. So we have dimethyl. So this is also a methyl group right over there. And when we think about numbering, we could number from one, two three. Or we could start numbering one, two, three. And in general, you want to go numbering in the direction where you hit the functional group first. So we want to go one, two, three, four, and five. So this right here is 5-ethoxy. And this is one, two dimethyl. These two combined, you would call this one comma two dimethyl. And then when you want to list them in order, the ethoxy would take precedence in alphabetical because the di you shouldn't count in the alphabetical order. This is just saying two methyls. So you really just want to look at the two of whatever you're talking about. M comes after e in alphabetical order. So this is going to be 5-ethoxy. Actually let me just rewrite the whole thing. So this is going to be 5-ethoxy 1,2-dimethylcyclohexanamine. And we're done. This was probably one of the longest words we've used in naming. But hopefully you see when you break down the different pieces, it actually makes sense that it represents this molecule.
C-Nitroso compounds
C-Nitroso compounds, such as nitrosobenzene, are typically prepared by oxidation of hydroxylamines:
- RNHOH + [O] → RNO + H2O
S-Nitroso compounds
S-Nitroso compounds (S-nitrosothiols) are typically prepared by condensation of a thiol and nitrous acid:[1]
- RSH + HONO → RSNO + H2O
O-Nitroso compounds
O-Nitroso compounds are similar to S-nitroso compounds, but are less reactive because the oxygen atom is less nucleophilic than the sulfur atom. The formation of an alkyl nitrite from an alcohol and nitrous acid is a common example:
- ROH + HONO → RONO + H2O
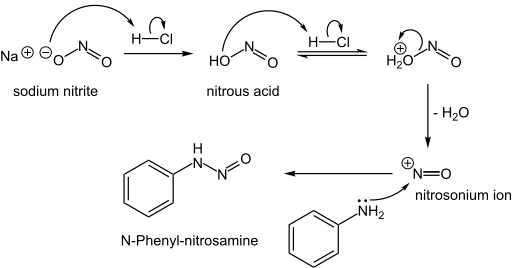
N-Nitrosamines
N-Nitrosamines, including the carcinogenic variety, arise from the reaction of nitrite sources with amino compounds, which can happen during the curing of meat. Typically, this reaction occurs when the nucleophilic nitrogen of a secondary amine attacks the nitrogen of the electrophilic nitrosonium ion:[2]
- NO2− + 2 H+ → NO+ + H2O
- R2NH + NO+ → R2N-NO + H+
Formation of an N-nitrosamine:
The nitrosamine can then lose water through protonation to form diazonium cation, which is a very useful intermediate to form different compounds.
References
- ^ Wang, P. G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A. J. (2002). "Nitric Oxide Donors: Chemical Activities and Biological Applications". Chemical Reviews. 102 (4): 1091–1134. doi:10.1021/cr000040l. PMID 11942788.
- ^ López-Rodríguez, Rocío; McManus, James A.; Murphy, Natasha S.; Ott, Martin A.; Burns, Michael J. (2020-09-18). "Pathways for N -Nitroso Compound Formation: Secondary Amines and Beyond". Organic Process Research & Development. 24 (9): 1558–1585. doi:10.1021/acs.oprd.0c00323. ISSN 1083-6160. S2CID 225483602.