| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C516441 |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H12O21P6 | |
| Molar mass | 605.984 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
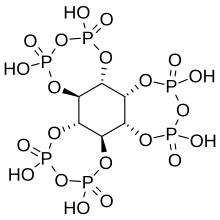
Myo-inositol trispyrophosphate (ITPP) is an inositol phosphate, a pyrophosphate, a drug candidate, and a putative performance-enhancing substance, which exerts its biological effects by increasing tissue oxygenation.[1]
Chemistry
ITPP is a pyrophosphate derivative of phytic acid with the molecular formula C6H12O21P6.[1]
Biological effects
ITPP is a membrane-permeant allosteric regulator of hemoglobin that mildly reduces its oxygen-binding affinity, which shifts the oxygen-hemoglobin dissociation curve to the right and thereby increases oxygen release from the blood into tissue.[1] Phytic acid, in contrast, is not membrane-permeant due to its charge distribution.[1]
Rodent studies in vivo demonstrated increased tissue oxygenation and dose-dependent increases in endurance during physical exercise, in both healthy mice and transgenic mice expressing a heart failure phenotype.[1]
The substance is believed to have a high potential for use in athletic doping, and liquid chromatography–mass spectrometry tests have been developed to detect ITPP in urine tests.[2] Its use as a performance-enhancing substance in horse racing has also been suspected and similar tests have been developed for horses[3]
ITPP has been studied for potential adjuvant use in the treatment of cancer in conjunction with chemotherapy, due to its effects in reducing tissue hypoxia.[4] Human clinical trials were registered in 2014 under the compound number OXY111A.[5] The substance has also been examined in the context of other illnesses involving hypoxia, such as cardiovascular disease and dementia[2]
See also
References
- ^ a b c d e Biolo, A; Greferath, R; Siwik, DA; Qin, F; Valsky, E; Fylaktakidou, KC; Pothukanuri, S; Duarte, CD; Schwarz, RP; Lehn, JM; Nicolau, C; Colucci, WS (2009). "Enhanced exercise capacity in mice with severe heart failure treated with an allosteric effector of hemoglobin, myo-inositol trispyrophosphate". Proc Natl Acad Sci U S A. 106 (6): 1926–1929. Bibcode:2009PNAS..106.1926B. doi:10.1073/pnas.0812381106. PMC 2644140. PMID 19204295.
- ^ a b Görgens, C; Guddat, S; Schänzer, W; Thevis, M (2014). "Screening and confirmation of myo-inositol trispyrophosphate (ITPP) in human urine by hydrophilic interaction liquid chromatography high resolution / high accuracy mass spectrometry for doping control purposes". Drug Test. Anal. 6 (11–12): 1102–1107. doi:10.1002/dta.1700. PMID 25070041.
- ^ Lam, G; Zhao, S; Sandhu, J; Yi, R; Loganathan, D; Morrissey, B (2014). "Detection of myo-inositol tris pyrophosphate (ITPP) in equine following an administration of ITPP". Drug Test. Anal. 6 (3): 268–276. doi:10.1002/dta.1473. PMID 23733541.
- ^ Limani, P; Linecker, M; Schneider, MA; Kron, P; Tschuor, C; Kachaylo, E; Ungethuem, U; Nicolau, C; Lehn, JM; Graf, R; Humar, B; Clavien, PA (2017). "The Allosteric Hemoglobin Effector ITPP Inhibits Metastatic Colon Cancer in Mice" (PDF). Ann. Surg. 266 (5): 746–753. doi:10.1097/SLA.0000000000002431. PMID 28742687. S2CID 20565432.
- ^ Limani, P; Linecker, M; Kron, P (2016). "Development of OXY111A, a novel hypoxia-modifier as a potential antitumor agent in patients with hepato-pancreato-biliary neoplasms - Protocol of a first Ib/IIa clinical trial". BMC Cancer. 16 (1): 812. doi:10.1186/s12885-016-2855-3. PMC 5070093. PMID 27756258.
