| |
| Names | |
|---|---|
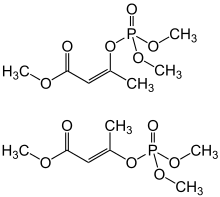
| IUPAC name
2-methoxycarbonyl-1-methylvinyl dimethyl phosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.177 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H13O6P | |
| Molar mass | 224.149 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 1.25 g/mL[2] |
| Melting point | 21 °C (70 °F; 294 K) (E isomer); 6.9 °C (Z isomer) |
| miscible[2] | |
| Vapor pressure | 0.003 mmHg (20°C)[2] |
| Hazards | |
| Flash point | 175 °C; 347 °F; 448 K[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3 mg/kg (rat, oral) 4 mg/kg (mouse, oral) 6-7 mg/kg (rat, oral)[3] |
LC50 (median concentration)
|
14 ppm (rat, 1 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.1 mg/m3 [skin][2] |
REL (Recommended)
|
TWA 0.01 ppm (0.1 mg/m3) ST 0.03 ppm (0.3 mg/m3) [skin][2] |
IDLH (Immediate danger)
|
4 ppm[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mevinphos is an organophosphate insecticide that acts as an acetylcholinesterase inhibitor to control insects in a wide range of crops. It is most commonly used for the control of chewing and sucking insects, as well as spider mites. Common synonym names are duraphos, fosdrin, menite, mevinfos, mevinox, phosdrin, and phosdrine. It is not allowed in the EU anymore.[4]
Manufacture
Mevinphos is produced by the reaction of trimethyl phosphite with chloroacetoacetate.[1]
References
- ^ a b Muller, Franz, ed. (2000). Agrochemicals: Composition, Production, Toxicology, Applications. Toronto: Wiley-VCH. ISBN 3-527-29852-5.
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0503". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Phosdrin". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Mevinphos in the Pesticide Properties DataBase (PPDB)
Further reading
- Wolverton, B.C., ed. (1975). Aquatic Plants for Removal of Mevinphos from the Aquatic Environment; Volume 72720 of NASA Technical memorandum. Mississippi: National Space Technology Laboratories (U.S.).
External links
- Mevinphos in the Pesticide Properties DataBase (PPDB)
