| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanediol[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | MADOL | ||
| 1730798 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.673 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH4O2 | |||
| Molar mass | 48.041 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 1.199 g/cm3[citation needed] | ||
| Boiling point | 194 °C (381 °F; 467 K) at 101 kPa[citation needed] | ||
| Vapor pressure | 16.1 Pa[citation needed] | ||
| Acidity (pKa) | 13.29[2] | ||
Refractive index (nD)
|
1.401[citation needed] | ||
| Hazards | |||
| Flash point | 99.753 °C (211.555 °F; 372.903 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
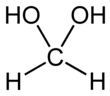
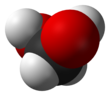
Methanediol, also known as formaldehyde monohydrate or methylene glycol, is an organic compound with chemical formula CH2(OH)2. It is the simplest geminal diol. In aqueous solutions it coexists with oligomers (short polymers). The compound is closely related and convertible to the industrially significant derivatives paraformaldehyde ((CH2O)n), formaldehyde (H2C=O), and 1,3,5-trioxane ((CH2O)3).[3]
Methanediol is a product of the hydration of formaldehyde. The equilibrium constant for hydration is estimated to be 103,[4]CH2(OH)2 predominates in dilute (<0.1%) solution. In more concentrated solutions, it oligomerizes to HO(CH2O)nH.[3]
YouTube Encyclopedic
-
1/5Views:3 2213 987706361 243384
-
Making Formaldehyde with a Copper Catalyst at home DIY
-
How to Name CH3SH (methanethiol)
-
3d Molecular structure of formic acid
-
How to Draw Bohr-Rutherford Diagrams - Potassium
-
PTS Chemistry Chapter 09
Transcription
Occurrence
The dianion, methanediolate, is believed to be an intermediate in the crossed Cannizzaro reaction.
Gaseous methanediols can be generated by electron irradiation and sublimation of a mixture of methanol and oxygen ices.[5]
Methanediol is believed to occur as an intermediate in the decomposition of carbonyl compounds in the atmosphere, and as a product of ozonolysis on these compounds.[5]
Safety
Methanediol, rather than formaldehyde, is listed as one of the main ingredients of "Brazilian blowout", a hair-straightening formula marketed in the United States. The equilibrium with formaldehyde has caused concern since formaldehyde in hair straighteners is a health hazard.[6][7] Research funded by the Professional Keratin Smoothing Council (PKSC), an industry association that represents selected manufacturers of professional-use only keratin smoothing products, has disputed the risk.[8]
See also
- Orthoformic acid (methanetriol)
- Orthocarbonic acid (methanetetrol)
References
- ^ "Methanediol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 20 October 2011.
- ^ Bell, R. P.; McTigue, P. T. (1960). "603. Kinetics of the aldol condensation of acetaldehyde". Journal of the Chemical Society (Resumed): 2983. doi:10.1039/JR9600002983.
- ^ a b Reuss, Günther; Disteldorf, Walter; Gamer, Armin Otto; Hilt, Albrecht (2000). "Formaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_619. ISBN 978-3527306732.
- ^ Eric V. Anslyn, Dennis A. Dougherty (2006), Modern physical organic chemistry. University Science Books. ISBN 1-891389-31-9. 1095 pages
- ^ a b Zhu, Cheng; Kleimeier, N. Fabian; Turner, Andrew M.; Singh, Santosh K.; Fortenberry, Ryan C.; Kaiser, Ralf I. (4 January 2022). "Synthesis of methanediol [CH 2 (OH) 2 ]: The simplest geminal diol". Proceedings of the National Academy of Sciences. 119 (1): e2111938119. Bibcode:2022PNAS..11911938Z. doi:10.1073/pnas.2111938119. PMC 8740743. PMID 34969838.
- ^ "Hair Smoothing Products That Could Release Formaldehyde". www.osha.gov. Occupational Safety and Health Administration.
- ^ SpecialChem. "Industry News".
- ^ Golden, R.; Valentini, M. (July 2014). "Formaldehyde and methylene glycol equivalence: Critical assessment of chemical and toxicological aspects". Regulatory Toxicology and Pharmacology. 69 (2): 178–186. doi:10.1016/j.yrtph.2014.03.007. PMID 24709515.