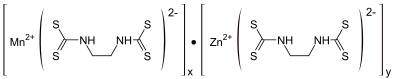
Mancozeb is a dithiocarbamate non-systemic agricultural fungicide with multi-site, protective action on contact. It is a combination of two other dithiocarbamates: maneb and zineb.[1] The mixture controls many fungal diseases in a wide range of field crops, fruits, nuts, vegetables, and ornamentals. It is marketed as Penncozeb, Trimanoc, Vondozeb, Dithane, Manzeb, Nemispot, and Manzane. In Canada, a mixture of zoxamide and mancozeb was registered for control of the mildew named Gavel as early as 2008.[2]
YouTube Encyclopedic
-
1/3Views:265 59554 44719 166
-
Propineb vs Mancozeb/Antracol vs M45/Who is Better/M45 Fungicide
-
Syngenta Abic Fungicide | Mancozeb 75% WP | M-45 | खरीदे | #TAACचैनल
-
ছত্রাকনাশক পরিচিতি পর্ব-২| ম্যানকোজেব/Mancozeb 75% কার্যকারিতা ও ব্যবহার বিধি। use of fungiside
Transcription
Mechanism
Mancozeb reacts with, and inactivates, the sulfhydryl groups of amino acids and enzymes within fungal cells, resulting in disruption of lipid metabolism, respiration, and production of adenosine triphosphate.[3]
Mancozeb is listed under FRAC code M:03 The "M:" refers to Chemicals with Multi-Site Activity. "M:" FRAC groups are defined as generally considered as a low risk group without any signs of resistance developing to the fungicides.[4]
Toxicology
A major toxicological concern is ethylenethiourea (ETU), an industrial contaminant and a breakdown product of mancozeb and other EBDC pesticides. It has potential to cause goiter, a condition in which the thyroid gland is enlarged and has produced birth defects and cancer in experimental animals. ETU has been classified as a probable human carcinogen by the EPA.[5] Mancozeb has been shown to have significant negative effects on beneficial root fungi - totally preventing spore germination at levels far below recommended dosage levels.[6]
See also
References
- ^ "Mancozeb". Cornell University. 1993. Retrieved 2014-07-20.
It is a combination of two other chemicals of this class, maneb and zineb
- ^ "Gowan buys Dow's Gavel potato fungicide". grainews.ca. July 18, 2008.
- ^ Tomlin C.D.S (2003). The Pesticide Manual - A world compendium (Thirteenth ed.). British Crop Protection Council.
- ^ "FRAC Code List ©*2017" (PDF). Fungicide Resistance Action Committee. Retrieved November 27, 2017.
- ^ http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/mancozeb-ext.html
- ^ https://www.beyondpesticides.org/assets/media/documents/Mycorrhizal_fungi_in_ecotoxicological_studies_Soil.pdf[bare URL PDF]
External links
- Mancozeb in the Pesticide Properties DataBase (PPDB)
