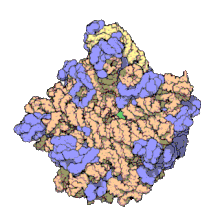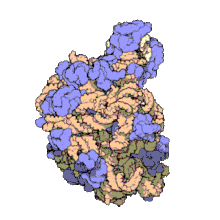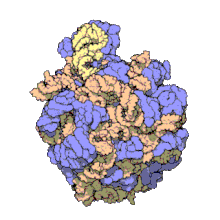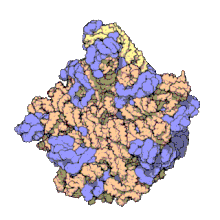
The term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially (i.e., with regard to their chemical shape), and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units (i.e., they are polymers). Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts (e.g., in supramolecular chemistry and nanotechnology). MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions (rather than covalent bonds), and can be in either non-repeating structures (e.g., as in the ribosome (image) and cell membrane architectures), or in repeating linear, circular, spiral, or other patterns (e.g., as in actin filaments and the flagellar motor, image). The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale (molecular dimensions) of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.

YouTube Encyclopedic
-
1/1Views:2 467
-
Phosphoanhydrides: Biochemical Energy Transfer
Transcription
Let me summarize the bioorganic phosphorus chemistry that we've studied up till now. First we began by looking at phosphoric acid and we saw that it was suitable to buffer the cell at a pH near 7. Second, we looked at the phosphomonoesters; in fact, throughout the semester we've seen examples of transforming hydroxyl groups through phosphorylation into phosphomonoesters. These are good leaving groups, then, for substitution and elimination chemistry. Third, we've looked at the phosphodiester linkage, and its resistance to hydrolysis makes it suitable for storing information through nucleic acids like DNA and RNA. And fourth, we want to look at the phosphoanhydride group, which is shown here, and we'll see that it's quite suitable for chemical potential or energy storage in the cell. Okay, let's take a look at the phosphoanhydride, which is formed by a dehydration condensation reaction from phosphoric acid. So at high temperatures, the loss of water generates the functional entity which joins two phosphorus groups through an oxygen. It has a structure that's quite analogous to the carboxylic anhydride that we've already encountered in which carbonyl is linked to another carbonyl through the oxygen group. It's also formed by a dehydration condensation reaction from carboxylic acids. We encountered carboxylic anhydrides when we talked about bock groups and putting them on amino acids, if you want to remember where you, ah, where you heard that. Alright, what are some of the properties of the phosphoanhydride? Well, for one thing it forms an excellent leaving group in substitution chemistry. For example, this bond between this carbon and oxygen group is broken to generate inorganic pyrophosphate, whose structure is shown here. It's often abbreviated as PPi; it's a structure you probably want to memorize, you'll see it a lot in biochemistry. It's accelerated; it's a reaction that is accelerated by chelation to divalent metal ions such as magnesium and zinc that really causes electron density to be polarized in this bond and facilitates this reaction. What else besides, ah, the substitution chemistry are the phosphoanhydrides capable of doing? Well, this is where they come in and play their role in energy storage. It's the reaction that's known as phosphoryl transfer, and so for example for the molecule of ATP, adenosine triphosphate whose structure is shown here, the energy storage part of the molecule is this portion, the triphosphate portion, the other part really is there to serve as a recognition element that can dock in enzyme active sites. Phosphoryl transfer takes place at either of the three phosphorous positions shown here. So for example, with an alcohol functional group, ROH, ROH could come in and in fact it would attack the γ phosphorous, breaking the indicated bond there to make ADP, adenosine diphosphate, and this phosphomonoester from the alcohol. That's one phosphoryl transfer reaction. A second type of phosphoryl transfer reaction also would involve an alcohol, ROH, but it would, ah, attack at the β phosphorous, breaking the indicated bond there. That would leave adenosine monophosphate, and attached to the alcohol would be the pyrophosphate functional group that's shown here. That's the second type of phosphoryl transfer. The third type of phosphoryl transfer involves a different kind of reaction. It actually takes place by attacking the α phosphorous here, breaking pyrophosphate, so PPi is lost, and what we have, then, is the organic residue or organic constituent R bound to the adenosine group and so we call this an acyl adenylate or adenylate derivative, acyl adenylate if the R group is actually a carboxylic acid. So rather than an alcohol, we would have the important O attached to this phosphorous, the α phosphorous, and we'll actually see an example of acyl adenylate, which is what nature uses to activate carboxylic acids in the synthesis of proteins. We'll see that in the next series of webcasts. So, these are three modes of phosphorylation, and what makes this phosphorylation transfer reaction so important for energy storage is this concept that this is a metastable functional group. It has a very large negative free energy. We'll actually look at some of the free energy values for phosphoryl transfer. However, kinetically, it's quite stable, meaning that this reaction doesn't take place very easily; in fact, it's that chelation through a divalent metal that triggers this reaction that lowers its activation barrier and turns it on to be kinetically labile. So in general, this cell can allow ATP to run around and not be hydrolyzed by water, it's resistant to hydrolysis much like phosphodiesters are resistant to hydrolysis, but it can be turned on and become quite reactive by the chelation to a divalent metal. So the combination of having a large driving force but being kinetically stable makes it what we call metastable, and that really is an ideal, ah, functional ability for energy storage. Let's look at some of the thermodynamics behind these phosphoryl transfer reactions. In the case of hydrolysis of a molecule we've already encountered before, phosphoenolpyruvate, where we end up breaking the phosphomonoester group, the resulting enol is transformed into a carbonyl. So, this carbon used to be this CH2, but there was a tautomerization along the way. And if you remember back when we talked about bond energies, that carbonyl group had a huge number of, ah, k, kilocalories associated with it and so this reaction does two things. Not only does it break the phosphomonoester group, but it also does this, ah, tautomerization which generates a large amount of energy. There's a huge thermodynamic driving force in phosphoenolpyruvate hydrolysis. Here's an example of a phosphoanhydride that's called a mixed anhydride. It's a mixed anhydride because it contains a carboxylic acid on the one half and on the other half it contains the phosphoric acid. The hydrolysis of this mixed anhydride gives a relatively large amount of energy in comparison to the other energies that we'll see, that's a pretty significant number; not quite as large as phosphoenolpyruvate, but it's definitely up there. Pyrophosphate itself, it's hydrolysis into two phosphate groups is about, ah, half of that or about 7.9 kilocalories per mol. ATP, if you wanna know its various modes of, ah, phosphorylation hydrolysis either by the hydrolysis at the ah, γ position or between the α and the β positions, you can see that it has similar amounts of energies associated with it and, ah, that are comparable to the last reaction that we looked at. And finally if we look at a typical phosphorylation hydrolysis with a monophosphate like an AMP, we see that it carries still with it a fairly large thermodynamic driving force, about half of that where we hydrolyze the phosphoanhydride, ah, type bond. So phosphomonoester hydrolysis versus the phosphoanhydride hydrolysis has a factor of two difference in kilocalories per mol of energy currency associated with it.
Biomolecular complex

A biomolecular complex, also called a biomacromolecular complex, is any biological complex made of more than one biopolymer (protein, RNA, DNA, [5] carbohydrate) or large non-polymeric biomolecules (lipid). The interactions between these biomolecules are non-covalent. [6] Examples:
- Protein complexes, some of which are multienzyme complexes: proteasome, DNA polymerase III holoenzyme, RNA polymerase II holoenzyme, symmetric viral capsids, chaperonin complex GroEL-GroES, photosystem I, ATP synthase, ferritin.
- RNA-protein complexes: ribosome, spliceosome, vault, SnRNP. Such complexes in cell nucleus are called ribonucleoproteins (RNPs).
- DNA-protein complexes: nucleosome.
- Protein-lipid complexes: lipoprotein.[7][8]
The biomacromolecular complexes are studied structurally by X-ray crystallography, NMR spectroscopy of proteins, cryo-electron microscopy and successive single particle analysis, and electron tomography. [9] The atomic structure models obtained by X-ray crystallography and biomolecular NMR spectroscopy can be docked into the much larger structures of biomolecular complexes obtained by lower resolution techniques like electron microscopy, electron tomography, and small-angle X-ray scattering. [10]
Complexes of macromolecules occur ubiquitously in nature, where they are involved in the construction of viruses and all living cells. In addition, they play fundamental roles in all basic life processes (protein translation, cell division, vesicle trafficking, intra- and inter-cellular exchange of material between compartments, etc.). In each of these roles, complex mixtures of become organized in specific structural and spatial ways. While the individual macromolecules are held together by a combination of covalent bonds and intramolecular non-covalent forces (i.e., associations between parts within each molecule, via charge-charge interactions, van der Waals forces, and dipole–dipole interactions such as hydrogen bonds), by definition MAs themselves are held together solely via the noncovalent forces, except now exerted between molecules (i.e., intermolecular interactions).[citation needed]
MA scales and examples
The images above give an indication of the compositions and scale (dimensions) associated with MAs, though these just begin to touch on the complexity of the structures; in principle, each living cell is composed of MAs, but is itself an MA as well. In the examples and other such complexes and assemblies, MAs are each often millions of daltons in molecular weight (megadaltons, i.e., millions of times the weight of a single, simple atom), though still having measurable component ratios (stoichiometries) at some level of precision. As alluded to in the image legends, when properly prepared, MAs or component subcomplexes of MAs can often be crystallized for study by protein crystallography and related methods, or studied by other physical methods (e.g., spectroscopy, microscopy).[citation needed]
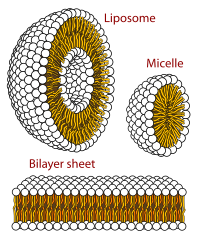

Virus structures were among the first studied MAs; other biologic examples include ribosomes (partial image above), proteasomes, and translation complexes (with protein and nucleic acid components), procaryotic and eukaryotic transcription complexes, and nuclear and other biological pores that allow material passage between cells and cellular compartments. Biomembranes are also generally considered MAs, though the requirement for structural and spatial definition is modified to accommodate the inherent molecular dynamics of membrane lipids, and of proteins within lipid bilayers.[15]
Virus assembly
During assembly of the bacteriophage (phage) T4 virion, the morphogenetic proteins encoded by the phage genes interact with each other in a characteristic sequence. Maintaining an appropriate balance in the amounts of each of these proteins produced during viral infection appears to be critical for normal phage T4 morphogenesis.[16] Phage T4 encoded proteins that determine virion structure include major structural components, minor structural components and non-structural proteins that catalyze specific steps in the morphogenesis sequence[17]
Research into MAs
The study of MA structure and function is challenging, in particular because of their megadalton size, but also because of their complex compositions and varying dynamic natures. Most have had standard chemical and biochemical methods applied (methods of protein purification and centrifugation, chemical and electrochemical characterization, etc.). In addition, their methods of study include modern proteomic approaches, computational and atomic-resolution structural methods (e.g., X-ray crystallography), small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS), force spectroscopy, and transmission electron microscopy and cryo-electron microscopy. Aaron Klug was recognized with the 1982 Nobel Prize in Chemistry for his work on structural elucidation using electron microscopy, in particular for protein-nucleic acid MAs including the tobacco mosaic virus (a structure containing a 6400 base ssRNA molecule and >2000 coat protein molecules). The crystallization and structure solution for the ribosome, MW ~ 2.5 MDa, an example of part of the protein synthetic 'machinery' of living cells, was object of the 2009 Nobel Prize in Chemistry awarded to Venkatraman Ramakrishnan, Thomas A. Steitz, and Ada E. Yonath.[18]
Non-biologic counterparts
Finally, biology is not the sole domain of MAs. The fields of supramolecular chemistry and nanotechnology each have areas that have developed to elaborate and extend the principles first demonstrated in biologic MAs. Of particular interest in these areas has been elaborating the fundamental processes of molecular machines, and extending known machine designs to new types and processes.[citation needed]
See also
- Multi-state modeling of biomolecules
- Quaternary structure
- Multiprotein complex
- Organelle: the broadest definition of "organelle" includes not only membrane bound cellular structures, but also very large biomolecular complexes.
- Multi-state modeling of biomolecules
References
- ^ Ban N, Nissen P, Hansen J, Moore PB, Steitz TA (August 2000). "The complete atomic structure of the large ribosomal subunit at 2.4 A resolution". Science. 289 (5481): 905–920. Bibcode:2000Sci...289..905B. CiteSeerX 10.1.1.58.2271. doi:10.1126/science.289.5481.905. PMID 10937989.
- ^ McClure W. "50S Ribosome Subunit". Archived from the original on 2005-11-24. Retrieved 2019-10-09.
- ^ Osborne AR, Rapoport TA, van den Berg B (2005). "Protein translocation by the Sec61/SecY channel". Annual Review of Cell and Developmental Biology. 21: 529–550. doi:10.1146/annurev.cellbio.21.012704.133214. PMID 16212506.
- ^ Legend, cover art, J. Bacteriol., October 2006.[full citation needed]
- ^ Kleinjung J, Fraternali F (July 2005). "POPSCOMP: an automated interaction analysis of biomolecular complexes". Nucleic Acids Research. 33 (Web Server issue): W342–W346. doi:10.1093/nar/gki369. PMC 1160130. PMID 15980485.
- ^ Moore PB (2012). "How should we think about the ribosome?". Annual Review of Biophysics. 41 (1): 1–19. doi:10.1146/annurev-biophys-050511-102314. PMID 22577819.
- ^ Neuman N (January 2016). "The Complex Macromolecular Complex". Trends in Biochemical Sciences. 41 (1): 1–3. doi:10.1016/j.tibs.2015.11.006. PMID 26699226.
- ^ Dutta S, Berman HM (March 2005). "Large macromolecular complexes in the Protein Data Bank: a status report". Structure. 13 (3): 381–388. doi:10.1016/j.str.2005.01.008. PMID 15766539.
- ^ Russell RB, Alber F, Aloy P, Davis FP, Korkin D, Pichaud M, et al. (June 2004). "A structural perspective on protein-protein interactions". Current Opinion in Structural Biology. 14 (3): 313–324. doi:10.1016/j.sbi.2004.04.006. PMID 15193311.
- ^ van Dijk AD, Boelens R, Bonvin AM (January 2005). "Data-driven docking for the study of biomolecular complexes". The FEBS Journal. 272 (2): 293–312. doi:10.1111/j.1742-4658.2004.04473.x. hdl:1874/336958. PMID 15654870. S2CID 20148856.
- ^ "Structure of Fluid Lipid Bilayers". Blanco.biomol.uci.edu. 2009-11-10. Retrieved 2019-10-09.
- ^ Experimental system, dioleoylphosphatidylcholine bilayers. The hydrophobic hydrocarbon region of the lipid is ~30 Å (3.0 nm) as determined by a combination of neutron and X-ray scattering methods; likewise, the polar/interface region (glyceryl, phosphate, and headgroup moieties, with their combined hydration) is ~15 Å (1.5 nm) on each side, for a total thickness about equal to the hydrocarbon region. See S.H. White references, preceding and following.
- ^ Wiener MC, White SH (February 1992). "Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure". Biophysical Journal. 61 (2): 434–447. Bibcode:1992BpJ....61..434W. doi:10.1016/S0006-3495(92)81849-0. PMC 1260259. PMID 1547331.
- ^ Hydrocarbon dimensions vary with temperature, mechanical stress, PL structure and coformulants, etc. by single- to low double-digit percentages of these values.[citation needed]
- ^ Gerle C (June 2019). "Essay on Biomembrane Structure". The Journal of Membrane Biology. 252 (2–3): 115–130. doi:10.1007/s00232-019-00061-w. PMC 6556169. PMID 30877332.
- ^ Floor E (February 1970). "Interaction of morphogenetic genes of bacteriophage T4". Journal of Molecular Biology. 47 (3): 293–306. doi:10.1016/0022-2836(70)90303-7. PMID 4907266.
- ^ Snustad DP (August 1968). "Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric". Virology. 35 (4): 550–63. doi:10.1016/0042-6822(68)90285-7. PMID 4878023.
- ^ "The Nobel Prize in Chemistry 2009". The Nobel Prize. Nobel Prize Outreach AB 2021. Retrieved 10 May 2021.
Further reading
General reviews
- Williamson JR (August 2008). "Cooperativity in macromolecular assembly". Nature Chemical Biology. 4 (8): 458–465. doi:10.1038/nchembio.102. PMID 18641626.
- Perrakis A, Musacchio A, Cusack S, Petosa C (August 2011). "Investigating a macromolecular complex: the toolkit of methods". Journal of Structural Biology. 175 (2): 106–12. doi:10.1016/j.jsb.2011.05.014. PMID 21620973.
- Dafforn TR (January 2007). "So how do you know you have a macromolecular complex?". Acta Crystallographica. Section D, Biological Crystallography. 63 (Pt 1): 17–25. doi:10.1107/S0907444906047044. PMC 2483502. PMID 17164522.
- Wohlgemuth I, Lenz C, Urlaub H (March 2015). "Studying macromolecular complex stoichiometries by peptide-based mass spectrometry". Proteomics. 15 (5–6): 862–79. doi:10.1002/pmic.201400466. PMC 5024058. PMID 25546807.
- Sinha C, Arora K, Moon CS, Yarlagadda S, Woodrooffe K, Naren AP (October 2014). "Förster resonance energy transfer - an approach to visualize the spatiotemporal regulation of macromolecular complex formation and compartmentalized cell signaling". Biochimica et Biophysica Acta (BBA) - General Subjects. 1840 (10): 3067–72. doi:10.1016/j.bbagen.2014.07.015. PMC 4151567. PMID 25086255.
- Berg JM, Tymoczko J, Stryer L (2002). Biochemistry (5th ed.). New York: W.H. Freeman. ISBN 978-0-7167-4955-4.
- Lehninger AL, Cox M, Nelson DL (2005). Lehninger principles of biochemistry (Fourth ed.). New York: W.H. Freeman. ISBN 978-0-7167-4339-2.
Reviews on particular MAs
- Valle M (May 2011). "Almost lost in translation. Cryo-EM of a dynamic macromolecular complex: the ribosome". European Biophysics Journal. 40 (5): 589–97. doi:10.1007/s00249-011-0683-6. PMID 21336521. S2CID 26027815.
- Monie TP (2017). "The Canonical Inflammasome: A Macromolecular Complex Driving Inflammation". Macromolecular Protein Complexes. Subcellular Biochemistry. Vol. 83. pp. 43–73. doi:10.1007/978-3-319-46503-6_2. ISBN 978-3-319-46501-2. PMID 28271472.
- Perino A, Ghigo A, Damilano F, Hirsch E (August 2006). "Identification of the macromolecular complex responsible for PI3Kgamma-dependent regulation of cAMP levels". Biochemical Society Transactions. 34 (Pt 4): 502–3. doi:10.1042/BST0340502. PMID 16856844.
Primary sources
- Lasker K, Förster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P, et al. (January 2012). "Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach". Proceedings of the National Academy of Sciences of the United States of America. 109 (5): 1380–1387. Bibcode:2012PNAS..109.1380L. doi:10.1073/pnas.1120559109. PMC 3277140. PMID 22307589.
- Russel D, Lasker K, Webb B, Velázquez-Muriel J, Tjioe E, Schneidman-Duhovny D, et al. (January 2012). "Putting the pieces together: integrative modeling platform software for structure determination of macromolecular assemblies". PLOS Biology. 10 (1): e1001244. doi:10.1371/journal.pbio.1001244. PMC 3260315. PMID 22272186.
- Barhoum S, Palit S, Yethiraj A (May 2016). "Diffusion NMR studies of macromolecular complex formation, crowding and confinement in soft materials". Progress in Nuclear Magnetic Resonance Spectroscopy. 94–95: 1–10. doi:10.1016/j.pnmrs.2016.01.004. PMID 27247282.
Other sources
- Nobel Prizes in Chemistry (2012), The Nobel Prize in Chemistry 2009, Venkatraman Ramakrishnan, Thomas A. Steitz, Ada E. Yonath, The Nobel Prize in Chemistry 2009, accessed 13 June 2011.
- Nobel Prizes in Chemistry (2012), The Nobel Prize in Chemistry 1982, Aaron Klug, The Nobel Prize in Chemistry 1982, accessed 13 June 2011.
External links
- Beck Group (2019), Structure and function of large macromolecular assemblies (Beck group home page), Beck Group - Structure and function of large molecular assemblies - EMBL, accessed 13 June 2011.
- DMA Group (2019), Dynamics of macromolecular assembly (DMA Group home page), Dynamics of Macromolecular Assembly Section | National Institute of Biomedical Imaging and Bioengineering, accessed 13 June 2011.
