 __ La3+ __ OH−
| |
| Names | |
|---|---|
| IUPAC name
Lanthanum(III) hydroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.994 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| La(OH)3 | |
| Molar mass | 189.93 g/mol |
| Ksp= 2.00·10−21 | |
| Structure | |
| hexagonal | |
| P63/m, No. 176 | |
a = 6.547 Å, c = 3.854 Å
| |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling:[1] | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Lanthanum(III) chloride |
Other cations
|
Cerium(III) hydroxide Actinium(III) hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lanthanum hydroxide is La(OH)
3, a hydroxide of the rare-earth element lanthanum.
YouTube Encyclopedic
-
1/2Views:1 4584 556
-
Lanthanum
-
Mod-01 Lec-38 Solar Cells
Transcription
Synthesis
Lanthanum hydroxide can be obtained by adding an alkali such as ammonia to aqueous solutions of lanthanum salts such as lanthanum nitrate. This produces a gel-like precipitate that can then be dried in air.[2]
- La(NO3)3 + 3 NH4OH → La(OH)3 + 3 NH4NO3
Alternatively, it can be produced by hydration reaction (addition of water) to lanthanum oxide.[3]
- La2O3 + 3 H2O → 2 La(OH)3
Characteristics
Lanthanum hydroxide does not react much with alkaline substances, however is slightly soluble in acidic solution.[2] In temperatures above 330 °C it decomposes into lanthanum oxide hydroxide (LaOOH), which upon further heating decomposes into lanthanum oxide (La2O3):[4]
- La(OH)3 LaOOH
- 2 LaOOH La2O3
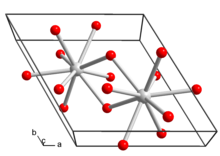
Lanthanum hydroxide crystallizes in the hexagonal crystal system. Each lanthanum ion in the crystal structure is surrounded by nine hydroxide ions in a tricapped trigonal prism.[5]
References
- ^ "C&L Inventory". echa.europa.eu.
- ^ a b E.V. Shkolnikov (2009). "Thermodynamic Characterization of the Amphoterism of Hydroxides and Oxides of Scandium Subgroup Elements in Aqueous Media". Russian Journal of Applied Chemistry. 82 (2): 2098–2104. doi:10.1134/S1070427209120040. S2CID 93220420.
- ^ Ding, Jiawen; Wu, Yanli; Sun, Weili; Li, Yongxiu (2006). "Preparation of La(OH)3 and La2O3 with Rod Morphology by Simple Hydration of La2O3". Journal of Rare Earths. 24 (4): 440–442. doi:10.1016/S1002-0721(06)60139-7.
- ^ Michael E. Brown, Patrick Kent Gallagher (2008). Handbook of Thermal Analysis and Calorimetry. Elsevier. p. 482. ISBN 978-0-44453123-0.
- ^ Beall, G.W.; Milligan, W.O.; Wolcott, Herbert A. (1977). "Structural trends in the lanthanide trihydroxides". Journal of Inorganic and Nuclear Chemistry. 39 (1): 65–70. doi:10.1016/0022-1902(77)80434-X.
External links
- "Lanthanum - Element information, properties and uses - Periodic Table". rsc.org.
- External MSDS 1
- External MSDS 2
- Lanthanum Oxide MSDS


![{\displaystyle \mathrm {{\xrightarrow[{-H_{2}O}]{330\ ^{o}C}}\ } }](https://wikimedia.org/api/rest_v1/media/math/render/svg/d68b2b30e45d08138b7179e5355cc2a4e39bb95b)
![{\displaystyle \mathrm {{\xrightarrow[{-H_{2}O}]{\Delta }}\ } }](https://wikimedia.org/api/rest_v1/media/math/render/svg/50d5d2823cc8d670b9962b2cf17adf45a830e8a1)