 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
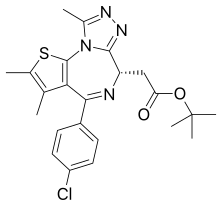
| Formula | C23H25ClN4O2S |
| Molar mass | 456.99 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
JQ1 is a thienotriazolodiazepine and a potent inhibitor of the BET family of bromodomain proteins which include BRD2, BRD3, BRD4, and the testis-specific protein BRDT in mammals. BET inhibitors structurally similar to JQ1 are being tested in clinical trials for a variety of cancers including NUT midline carcinoma.[1] It was developed by the James Bradner laboratory at Brigham and Women's Hospital and named after chemist Jun Qi. The chemical structure was inspired by patent of similar BET inhibitors by Mitsubishi Tanabe Pharma [WO/2009/084693]. Structurally it is related to benzodiazepines. While widely used in laboratory applications, JQ1 is not itself being used in human clinical trials because it has a short half life.
YouTube Encyclopedic
-
1/2Views:51 62815 201
-
Jay Bradner: Open-source cancer research
-
TEDxBoston - Dr Jay Bradner - Opensource Drug Discovery
Transcription
Efficacy in mouse models of cancer
Interest in JQ1 as a cancer therapeutic stemmed from its ability to inhibit BRD4 and BRD3, both of which form fusion oncogenes that drive NUT midline carcinoma.[2][3] Subsequent work demonstrated that a number of cancers including some forms of acute myelogenous leukemia (AML), multiple myeloma (MM) and acute lymphoblastic leukemia (ALL) were also highly sensitive to BET inhibitors.[4][5]
In other applications
JQ1 has also been investigated for other applications in the treatment of HIV infection,[6] as a male contraceptive,[7] and in slowing the progression of heart disease.[8]
See also
References
- ^ "Studies found for: bet inhibitor". ClinicalTrials.Gov. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services.
- ^ Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. (December 2010). "Selective inhibition of BET bromodomains". Nature. 468 (7327): 1067–73. Bibcode:2010Natur.468.1067F. doi:10.1038/nature09504. PMC 3010259. PMID 20871596.
- ^ Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, et al. (April 2011). "Differentiation of NUT midline carcinoma by epigenomic reprogramming". Cancer Research. 71 (7): 2686–96. doi:10.1158/0008-5472.CAN-10-3513. PMC 3070805. PMID 21447744.
- ^ Belkina AC, Denis GV (June 2012). "BET domain co-regulators in obesity, inflammation and cancer". Nature Reviews. Cancer. 12 (7): 465–77. doi:10.1038/nrc3256. PMC 3934568. PMID 22722403.Shi J, Vakoc CR (June 2014). "The mechanisms behind the therapeutic activity of BET bromodomain inhibition". Molecular Cell. 54 (5): 728–36. doi:10.1016/j.molcel.2014.05.016. PMC 4236231. PMID 24905006.
- ^ Da Costa D, Agathanggelou A, Perry T, Weston V, Petermann E, Zlatanou A, et al. (July 2013). "BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia". Blood Cancer Journal. 3 (7): e126. doi:10.1038/bcj.2013.24. PMC 3730202. PMID 23872705.
- ^ Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, et al. (December 2012). "BET bromodomain inhibition as a novel strategy for reactivation of HIV-1". Journal of Leukocyte Biology. 92 (6): 1147–54. doi:10.1189/jlb.0312165. PMC 3501896. PMID 22802445.
- ^ Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE, et al. (August 2012). "Small-molecule inhibition of BRDT for male contraception". Cell. 150 (4): 673–84. doi:10.1016/j.cell.2012.06.045. PMC 3420011. PMID 22901802.
- ^ Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, et al. (August 2013). "BET bromodomains mediate transcriptional pause release in heart failure". Cell. 154 (3): 569–82. doi:10.1016/j.cell.2013.07.013. PMC 4090947. PMID 23911322.
