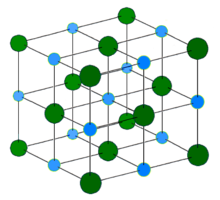
In chemistry, an ionic crystal is a crystalline form of an ionic compound. They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Examples of such crystals are the alkali halides, including potassium fluoride (KF), potassium chloride (KCl), potassium bromide (KBr), potassium iodide (KI), sodium fluoride (NaF).[1] Sodium chloride (NaCl) has a 6:6 co-ordination. The properties of NaCl reflect the strong interactions that exist between the ions. It is a good conductor of electricity when molten, but very poor in the solid state. When fused the mobile ions carry charge through the liquid.[2] They are characterized by strong absorption of infrared radiation and have planes along which they cleave easily. The exact arrangement of ions in an ionic lattice varies according to the size of the ions in the solid.[3]
YouTube Encyclopedic
-
1/3Views:154 60613 7696 163
-
Free Atmospheric Electricity Powers Small Motor - Tesla Radiant Energy
-
Lemurian Energy Staff- zero point, orgonite, radionics, reiki, massage, healing
-
Energy pendant Energy Transfer Test www 1000eco com
Transcription
References
- ^ "Chemicals of the natural environment" (PDF). Retrieved 1 February 2008.[dead link]
- ^ "Ionic Structures". Retrieved 1 February 2008.
- ^ "Chemicals of the natural environment" (PDF). Retrieved 1 February 2008.[dead link]
External links
- Art of the States: Anea musical work inspired by ionic crystals
