| |
| Names | |
|---|---|
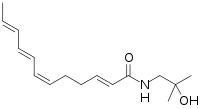
| Preferred IUPAC name
(2E,6Z,8E,10E)-N-(2-Hydroxy-2-methylpropyl)dodeca-2,6,8,10-tetraenamide | |
| Other names
Hydroxy-α-sanshool
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C16H25NO2 | |
| Molar mass | 263.381 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxy-alpha-sanshool is a molecule found in plants from the genus Zanthoxylum. It is believed to be responsible for the numbing and tingling sensation caused by eating food cooked with Sichuan peppercorns and Uzazi.
The term sanshool in the compound's name is derived from the Japanese term for the Japanese pepper, sanshō (山椒) (literally, mountain pepper), to which was appended the suffix -ol, indicating an alcohol.
Mechanism
The chemical structure of hydroxy-alpha-sanshool resembles capsaicin as both are fatty acid amides, but the mechanism of action by which it induces nerve sensations has been a matter of debate. Like capsaicin, hydroxy-alpha-sanshool is an agonist at the pain integration channels TRPV1 and TRPA1; however, evidence suggests that the inhibition of tandem pore domain potassium channels KCNK3, KCNK9, and KCNK18 are primarily responsible for sanshool's effects.[1]
Hydroxy-alpha-sanshool excites D-hair afferent nerve fibers, a distinct subset of the sensitive light touch receptors in the skin, and targets novel populations of Aβ and C-fiber nerve fibers.[2]
Extraction
To isolate the molecule from the pepper in form of an extract, steam distillation can be used: Dried peels of the fruit are immersed in a mixture of lower alcohols (for example ethanol) and water with a mass percentage between 35% and 65% of the alcohol. The solution gets heated up in the process of steam distillation where the aqueous part evaporates and takes parts of the hydroxy-alpha- sanshool up, too. The distillate separates in two phases: the aqueous ethanol phase and the oil phase which contains the desired molecule.
Steam distillation extraction methods demonstrate yields of approximately 60%.[3]
See also
References
- ^ Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D (July 2008). "Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels". Nat. Neurosci. 11 (7): 772–9. doi:10.1038/nn.2143. PMC 3072296. PMID 18568022.
- ^ Lennertz, Richard C; Tsunozaki, Makoto; Bautista, Diana M; Stucky, Cheryl L (24 Mar 2010). "Physiological basis of tingling paresthesia evoked by hydroxy-α-sanshool". J. Neurosci. Society for Neuroscience. 30 (12): 4353–4361. doi:10.1523/JNEUROSCI.4666-09.2010. PMC 2852189. PMID 20335471.
- ^ Schlander, David; Schleif, Chiara; Schnell, Maxim; Roumeliotis, Paul. "Hydroxy-alpha-sanshool" (PDF). Technische Universität Darmstadt: 2.
{{cite journal}}: Cite journal requires|journal=(help)
