| |||
| Names | |||
|---|---|---|---|
| Other names
Iron cytoporphyrin IX, formilporphyrin
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| MeSH | Heme+a | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C49H56O6N4Fe | |||
| Molar mass | 852.837 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
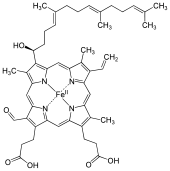
Heme A (or haem A) is a heme, a coordination complex consisting of a macrocyclic ligand called a porphyrin, chelating an iron atom. Heme A is a biomolecule and is produced naturally by many organisms. Heme A, often appears a dichroic green/red when in solution, is a structural relative of heme B, a component of hemoglobin, the red pigment in blood.
YouTube Encyclopedic
-
1/5Views:4412 2387 64647318 361
-
Porphyrins | Processing of Heme b to Heme a & Heme c
-
Chlorophyll vs Heme - a visual comparison / an Ode to Plants
-
Heme Synthesis (Heme/Onc) - USMLE Step 1
-
Pigments Porphyrins Cytochromes, Chlorophyll and Heme
-
Porphyrins | Heme b Biosynthesis and its Regulation
Transcription
Relationship to other hemes
Heme A differs from heme B in that a methyl side chain at ring position 8 is oxidized to a formyl group and a hydroxyethylfarnesyl group, an isoprenoid chain, has been attached to the vinyl side chain at ring position 2 of the iron tetrapyrrole heme. Heme A is similar to heme o, in that both have this farnesyl addition at position 2 but heme O does not have the formyl group at position 8, still containing the methyl group. The correct structure of heme A, based upon NMR and IR experiments of the reduced, Fe(II) form of the heme, was published in 1975.[1] The structure was confirmed by synthesis of the dimethyl ester of the iron-free form.[2]
History
Heme A was first isolated by the German biochemist Otto Warburg in 1951 and shown by him to be the active component of the integral membrane metalloprotein cytochrome c oxidase.[3]
Stereochemistry
The final structural question of the exact geometric configuration about the first carbon at ring position 3 of ring I, the carbon bound to the hydroxyl group, has been shown to be the chiral S configuration.[4]
Like heme B, heme A is often attached to the apoprotein through a coordinate bond between the heme iron and a conserved amino acid side-chain. In the important respiratory protein cytochrome c oxidase (CCO) this ligand 5 for the heme A at the oxygen reaction center is a histidyl group.[5] Histidine is a common ligand for many hemeproteins including hemoglobin and myoglobin.
Heme A in the cytochrome a portion of cytochrome c oxidase, bound by two histidine residues (shown in pink)[6]
An example of a metalloprotein that contains heme A is cytochrome c oxidase. This very complicated protein contains heme A at two different sites, each with a different function. The iron of the heme A of cytochrome a is hexacoordinated, that is bound with 6 other atoms. The iron of the heme A of cytochrome a3 is sometimes bound by 5 other atoms leaving the sixth site available to bind dioxygen (molecular oxygen).[6] In addition, this enzyme binds 3 copper, magnesium, zinc, and several potassium and sodium ions. The two heme A groups in CCO are thought to readily exchange electrons between each other, the copper ions and the closely associated protein cytochrome c.
Both the formyl group and the isoprenoid side chain are thought to play important roles in conservation of the energy of oxygen reduction by cytochrome c oxidase. CCO is thought to be responsible for conserving the energy of dioxygen reduction by pumping protons into the inter-membrane mitochondrial space. Both the formyl and hydroxyethylfarnesyl groups of heme A are thought to play important roles in this critical process, as published by the influential group of S. Yoshikawa.[7]
See also
- Heme
- Hemoprotein
- Cytochrome c oxidase (Complex IV of cellular respiration)
References
- ^ Caughey, W.S.; Smythe, G.A.; O'Keefe, D.H.; Maskasky, J.E.; Smith, M.L. (1975). "Heme A of Cytochrome c Oxidase". Journal of Biological Chemistry. 250 (19): 7602–7622. doi:10.1016/S0021-9258(19)40860-0. PMID 170266.
- ^ Battersby, Alan R.; McDonald, Edward; Thompson, Mervyn; Chaudhry, Irshad A.; Clezy, Peter S.; Fookes, Christopher J. R.; Hai, Ton That (1985). "Isolation, crystallisation, and synthesis of the dimethyl ester of porphyrin a, the iron-free prosthetic group of cytochrome c oxidase". Journal of the Chemical Society, Perkin Transactions 1: 135. doi:10.1039/P19850000135.
- ^ Warburg, O; Gewitz H S. (1951). "Cytohämin aus Herzmuskel". Zeitschrift für Physiologische Chemie. 288 (1): 1–4. doi:10.1515/bchm2.1951.288.1.1. PMID 14860765.
- ^ Yamashita E, Aoyama H, Yao M, et al. (2005). "Absolute configuration of the hydroxyfarnesylethyl group of heme A, determined by X-ray structural analysis of bovine heart cytochrome c oxidase using methods applicable at 2.8 Angstrom resolution". Acta Crystallographica D. 61 (10): 1373–1377. doi:10.1107/S0907444905023358. PMID 16204889.
- ^ Tsukihara T, Shimokata K, Katayama Y, et al. (2003). "The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process". PNAS. 100 (26): 15304–15309. Bibcode:2003PNAS..10015304T. doi:10.1073/pnas.2635097100. PMC 307562. PMID 14673090.
- ^ a b Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; et al. (1998). "Redox-Coupled Crystal Structural Changes in Bovine Heart Cytochrome c Oxidase". Science. 280 (5370): 1723–1729. doi:10.1126/science.280.5370.1723. PMID 9624044. S2CID 37147458.
- ^ Shimokata K, Katayama Y, Murayama H, et al. (2007). "The proton pumping pathway of bovine heart cytochrome c oxidase". PNAS. 104 (10): 4200–4205. Bibcode:2007PNAS..104.4200S. doi:10.1073/pnas.0611627104. PMC 1820732. PMID 17360500.