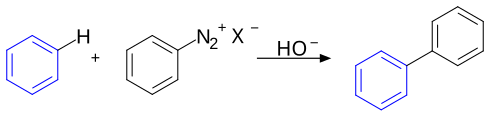| Gomberg–Bachmann reaction | |
|---|---|
| Named after | Moses Gomberg Werner Emmanuel Bachmann |
| Reaction type | Coupling reaction |
The Gomberg–Bachmann reaction, named for the Russian-American chemist Moses Gomberg and the American chemist Werner Emmanuel Bachmann, is an aryl-aryl coupling reaction via a diazonium salt.[1][2][3]
The arene compound (here benzene) is reacted with a diazonium salt in the presence of a base to provide the biaryl through an intermediate aryl radical. For example, p-bromobiphenyl may be prepared from 4-bromoaniline and benzene:[4]
- BrC6H4NH2 + C6H6 → BrC6H4−C6H5
The reaction offers a wide scope for both diazonium component and arene component but yields are generally low following the original procedure (less than 40%), given the many side-reactions of diazonium salts. Several improvements have been suggested. One possibility is to employ diazonium tetrafluoroborates in arene solvent together with a phase-transfer catalyst,[5] another is to use 1-aryl-3,3-dialkyltriazenes.[6]
Pschorr reaction
One intramolecular variation which gives better results is the Pschorr cyclization:[7][8][9]
The group Z can be CH2, CH2CH2, NH and CO (to fluorenone[10]) to name just a few.
See also
References
- ^ Gomberg, M.; Bachmann, W. E. (1924). "The Synthesis of Biaryl Compounds by Means of the Diazo Reaction". J. Am. Chem. Soc. 42 (10): 2339–2343. doi:10.1021/ja01675a026.
- ^ W. Pötsch. Lexikon bedeutender Chemiker (VEB Bibliographisches Institut Leipzig, 1989) (ISBN 3817110553)
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ M. Gomberg; W. E. Bachmann (1928). "p-Bromobiphenyl". Organic Syntheses. 8: 42. doi:10.15227/orgsyn.008.0042.; Collective Volume, vol. 1, p. 113
- ^ J. R. Beadle, S. H. Korzeniowski, D .E. Rosenberg, B. J. Garcia-Slanga, G. W. Gokel; Korzeniowski; Rosenberg; Garcia-Slanga; Gokel (1984). "Phase-transfer-catalyzed Gomberg-Bachmann synthesis of unsymmetrical biarenes: a survey of catalysts and substrates". J. Org. Chem. 49 (9): 1594–603. doi:10.1021/jo00183a021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ T. B. Patrick, R. P. Willaredt, D. J. DeGonia (1985). "Synthesis of biaryls from aryltriazenes". J. Org. Chem. 50 (13): 2232–2235. doi:10.1021/jo00213a007.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pschorr, Robert (1896). "Neue Synthese des Phenanthrens und seiner Derivate" [New Synthesis of Phenanthrene and Its Derivatives]. Chem. Ber. (in German). 29 (1): 496–501. doi:10.1002/cber.18960290198.
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Laali, Kenneth K.; Shokouhimehr, Mohammadreza (2009). "The Pschorr Reaction, a Fresh Look at a Classical Transformation". Current Organic Synthesis. 6 (2): 193–202. doi:10.2174/157017909788167275.
- ^ Stephen A. Chandler; Peter Hanson; Alec B. Taylor; Paul H. Walton; Allan W. Timms (2001). "Sandmeyer reactions. Part 5.1 Estimation of the rates of 1,5-aryl/aryl radical translocation and cyclisation during Pschorr fluorenone synthesis with a comparative analysis of reaction energetics". J. Chem. Soc., Perkin Trans. 2 (2): 214–228. doi:10.1039/b006184k.