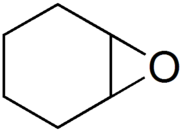
| |
| Names | |
|---|---|
| IUPAC name
7-Oxabicyclo[4.1.0]heptane
| |
| Other names
Epoxycyclohexane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.462 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10O | |
| Molar mass | 98.145 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 0.97 g·cm−3[1] |
| Melting point | ca. -40 °C[1] |
| Boiling point | ca. 130 °C[1] |
| Practically insoluble[1] | |
| Vapor pressure | 12 mbar (at 20 °C)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyclohexene oxide is a cycloaliphatic epoxide. It can react in cationic polymerization to poly(cyclohexene oxide). As cyclohexene is monovalent, poly(cyclohexene oxide) is a thermoplastic.
YouTube Encyclopedic
-
1/3Views:148 0001 45375 855
-
Cyclic ethers and epoxide naming | Organic chemistry | Khan Academy
-
Cyclohexene
-
Ring-opening Sn2 reaction of expoxides
Transcription
In the last video, we named some fairly simple ethers. In this video, we're going to think about slightly more complicated ones. In particular, what happens if, in the process of having an ether, we actually have a ring as opposed to just a long chain? So you can imagine a molecule that looks something like this. You have your oxygen. On this side of the oxygen, you have this carbon chain right here. You have a carbon chain like this. But then that chain bonds back to the oxygen. So we have a ring here. It's not obvious how to name this. You can't just look it this side and call it methyl. And then that side, and call it a methyl as well. It's the same side. It connects back to itself. How do you name this type of ether? What you do is, you just number it. You number the longest carbon chain, like we've always done in the case of an alkane. We can start numbering here. 1, 2, 3, 4. If we just think about the carbon chain by itself. We know if it's one carbon, the prefix is meth-. Two, it's eth-. Three, it's prop-. Four, it's but-. So if this was just a carbon chain, we would call this butane. If we only looked at this carbon chain right here, you would call this butane. But obviously this isn't butane. We have this oxygen that's bonding to the 1 and 4 carbons of the butane. To make that clear, we call this-- Let me color code this part right here, this oxygen right there. It's bonded to the 1 and the 4 carbon. So we call this 1 comma 4. And this is our new word that we're going to learn in this video. 1,4-epoxybutane. And it doesn't just apply when the ether forms a large ring. It can actually form a little subset ring on a regular chain. So you could imagine something like this. Let me draw a chain of carbons. Let's say we have five carbons. 1, 2, 3, 4, 5, just like that. Let's say that between this carbon and this carbon, instead of having a double bond, this carbon actually bonds to an oxygen, which then bonds to this carbon over here. Obviously, every carbon has four bonds, the ones that we're not drawing, those are hydrogens. How do we name this? Well, same exact process. We actually start numbering the chain closer to where the oxygen is bonded. So we start numbering at this end over here. 1, 2, 3, 4, 5. So this is pentane. The oxygen is bonded to the 1 and the 2 carbons. So we call this 1,2-epoxypentane. 1 comma 2-epoxypentane. Now, in the last video, I told you that, in general, ethers are fairly nonreactive. They actually make for good solvents. But, what I've just drawn here is a special case of ethers called epoxides. When you just have this three atom chain right here, where it's two carbons and an oxygen. This is a special case of an ether called an epoxide. This is called an epoxide. And this, unlike most ethers, is very reactive. Another way you could think about it, it's very unstable. This is very reactive. Sometimes people consider these separate from ethers. The reason why they're very reactive, is this three member ring right here. There's a lot of strain on these bonds. These electrons, these bonds don't like to be that close to each other. If you actually tried to make it with an actual model set with molecules, you would have trouble making it bend enough to actually make this bond. So this is highly, highly, highly unstable. There's actually an alternate way to name epoxides. The alternate way, so this is a completely legitimate way. You could name it just like an ether with a ring. This is 1,2-epoxypentane. But the alternate way is to pretend like you had a double bond here. That instead of this oxygen here, you had a double bond. If you had a double bond here, this thing would be called, depending how you want to name it, it could be called 1-pentene. That's if there was not this oxygen here, but if there was a double bond here. 1-pentene would look like this. 1, 2, 3, 4, 5. This is the 1 carbon. So, 1, 2, 3, 4, 5. This is what 1-petene looks like. We've learned that many, many, many videos ago. Sometimes it's called pent-1-ene, depending on which convention. This is the more common one. We have this oxygen here, instead of this double bond. Instead of calling it just 1-pentene, we call it 1-pentene oxide. Just like that. So both of these are the names for the same exact molecule. This makes it clear that it's an epoxide. That's kind of the special ether that is more reactive. This is just the general way that we name any type of cyclic ether. So let's just do one more just to make the point clear. Let's have a cycle branching off of a cycle. Let's have an epoxide off of another ring. Just to make the point clear. These aren't too hard to name. But the first time you seen them, a little daunting. Let's say we have a cyclohexane ring right here. So this is cyclohexane. But let's say we have a little epoxy branching off of it, just like this. We have that going on. If we wanted to make it clear that this is an epoxide, we would essentially pretend. First pretend that this is just a double bond. If this was just a double bond, this would be cyclohexene. If this oxygen wasn't there, and instead we just had a double bond here. You actually don't have to specify the number when you only have one double bonded cyclohexene. Because it could have been anywhere, and it would have essentially been the same molecule. But since we have this oxygen here, instead of a double bond that's bonding to both of these carbons, we call this cyclohexene oxide. This part, right here, makes us name this cyclohexene oxide. Or if we wanted to just name this as a traditional ether, we would just name this cyclohexane and put the epoxy in front of it. Either of these are valid. Once again, you don't have to number it. Because you could call it, 1,2-epoxycyclohexane, if you made this the 1 or the 2 carbon. But you know it's going to be on adjacent carbons. And it could have really been on any of these two. It could have been on the 3 and the 4, and it would have essentially been the same molecule. So this actually makes it clear exactly what the molecular structure of the molecule is. So anyway, I thought you would enjoy that. And in the next video, I told you that epoxides are reactive. So I'll actually show you a reaction dealing with epoxides.
Production
Cyclohexene oxide is produced in epoxidation reaction from cyclohexene. The epoxidation can take place either in a homogeneous reaction by peracids[2] or heterogeneous catalysis (e.g. silver and molecular oxygen).[3][4][5]
In industrial production the heterogeneously catalyzed synthesis is preferred because of better atom economy, a simpler separation of the product and easier recycling of catalyst. A short overview and an investigation of the oxidation of cyclohexene by hydrogen peroxide is given in the literature.[6] In recent times the catalytic oxidation of cyclohexene by (immobilized) metalloporphyrin complexes has been found to be an efficient way.[7][8]
In laboratory, cyclohexene oxide can also be prepared by reacting cyclohexene with magnesium monoperoxyphthalate (MMPP) in a mixture of isopropanol and water as solvent at room temperature.[9]
With this method, good yields up to 85 % can be reached.
Properties and reactions
Cyclohexene has been studied extensively by analytical methods.[10] Cyclohexene oxide can be polymerized in solution, catalyzed by a solid acid catalyst.[11]
References
- ^ a b c d e f Record of Epoxycyclohexane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 1 February 2014.
- ^ M. Quenard; V. Bonmarin; G. Gelbard (1987). "Epoxidation of olefins by hydrogen peroxide catalyzed by phosphonotungstic complexes". Tetrahedron Letters. 28 (20): 2237–2238. doi:10.1016/S0040-4039(00)96089-1.
- ^ Ha Q. Pham; Maurice J. Marks (2005), "Epoxy Resins", Ullmann's Encyclopedia of Industrial Chemistry (in German), doi:10.1002/14356007.a09_547.pub2, ISBN 3527306730
- ^ Siegfried Rebsdat; Dieter Mayer (2001), "Ethylene Oxide", Ullmann's Encyclopedia of Industrial Chemistry (in German), doi:10.1002/14356007.a10_117, ISBN 3527306730
- ^ Morazzoni, Franca; Canevali, Carmen; d'Aprile, Fiorenza; Bianchi, Claudia L.; Tempesti, Ezio; Giuffrè, Luigi; Airoldi, Giuseppe (1995). "Spectroscopic investigation of the molybdenum active sites on MoVI heterogeneous catalysts for alkene epoxidation". Journal of the Chemical Society, Faraday Transactions. 91 (21): 3969–3974. doi:10.1039/FT9959103969.
- ^ Ambili, V K; Dr.Sugunan, S (April 2011), Faculty of Sciences (ed.), Studies on Catalysis by Ordered Mesoporous SBA-15 Materials Modified with Transition Metals (in German), Cochin University of Science and Technology, retrieved 2014-07-27
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Costa, Andréia A. Ghesti; Grace F. de Macedo; Julio L. Braga; Valdeilson S. Santos; Marcello M. Dias; José A. Dias; Sílvia C.L. (2008). "Immobilization of Fe, Mn and Co tetraphenylporphyrin complexes in MCM-41 and their catalytic activity in cyclohexene oxidation reaction by hydrogen peroxide". Journal of Molecular Catalysis A: Chemical. 282 (1–2): 149–157. doi:10.1016/j.molcata.2007.12.024.
- ^ Xian-Tai Zhou; Hong-Bing Ji; Jian-Chang Xu; Li-Xia Pei; Le-Fu Wang; Xing-Dong Yao (2007). "Enzymatic-like mediated olefins epoxidation by molecular oxygen under mild conditions". Tetrahedron Letters. 48 (15): 2691–2695. doi:10.1016/j.tetlet.2007.02.066.
- ^ Brougham, Paul; Cooper, Mark S.; Cummerson, David A.; Heaney, Harry; Thompson, Nicola (1987). "Oxidation Reactions Using Magnesium Monoperphthalate: A Comparison with m-Chloroperoxybenzoic Acid". Synthesis. 1987 (11): 1015–1017. doi:10.1055/s-1987-28153. Retrieved 2020-07-31.
- ^ RM Ibberson; O. Yamamuro; I. Tsukushi (2006). "The crystal structures and phase behaviour of cyclohexene oxide". Chemical Physics Letters. 423 (4–6): 454–458. Bibcode:2006CPL...423..454I. doi:10.1016/j.cplett.2006.04.004.
- ^ Ahmed Yahiaoui; Mohammed Belbachir; Jeanne Claude Soutif; Laurent Fontaine (2005). "Synthesis and structural analyses of poly(1,2-cyclohexene oxide) over solid acid catalyst". Materials Letters. 59 (7): 759–767. doi:10.1016/j.matlet.2004.11.017.


