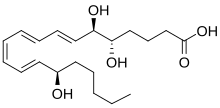
Epi-lipoxins are trihydroxy (i.e. containing 3 hydroxyl residues) metabolites of arachidonic acid. They are 15R-epimers of their lipoxin counterparts; that is, the epi-lipoxins, 15-epi-lipoxin A4 (15-epi-LxA4) and 15-epi-lipoxin B4 (15-epi-LXB4), differ from their respective lipoxin A4 (LxA4) and lipoxin B4 (LxB4) epimers in that their 15-hydroxy residue has R rather than S chirality. Formulae for these lipoxins (Lx) are:
- LxA4: 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-ETE
- LxB4: 5S,14R,15S-trihydroxy-6E,8Z,10E,12E-ETE
- 15-epi-LxA4: 5S,6R,15R-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
- 15-epi-LxB4: 5S,14R,15R-trihydroxy-6E,8Z,10E,12E-eicosatrienoic acid
The two-epi-Lx's as well as the two lx's are nonclassic eicosanoids that, like other members of the specialized pro-resolving mediators class of autocoids, form during and act to resolve inflammatory responses.[1][2][3] Synthesis of the lipoxins typically involves a lipoxygenase enzyme which acts to add a 15S-hydroxyl residue to the lipoxin precursor, arachidonic acid, whereas synthesis of the epi-lipoxins involves aspirin-pretreated cyclooxygenase 2 or a cytochrome P450 enzyme which adds a 15R-hydroxyl residue to arachidonic acid.[4] In acknowledgement of the role played by aspirin-treated cyclooxygenase 2 in forming these products, the epi-lipoxins are sometimes termed ATL which stands for Aspirin-Triggered Lipoxins.
The counter-regulatory role of the epi-lipoxins in serving as stop signals for diverse inflammation responses is detailed at the lipoxin site.
YouTube Encyclopedic
-
1/3Views:620321667
-
වේදනානාශක ක්රියාකරන්නේ මෙහෙමයි | How Painkillers Work? | Explained in Sinhala
-
PATHO; Lecture 22 Inflammatory mediators 2
-
Free Lockdown Webinar Series - Auflösung chronischer Entzündungen Resolution of Chronic Inflammation
Transcription
See also
References
- ^ Serhan CN (2001). "Lipoxins and aspirin-triggered 15-epi-lipoxins are endogenous components of antiinflammation: emergence of the counterregulatory side". Arch. Immunol. Ther. Exp. (Warsz.). 49 (3): 177–88. PMID 11478391.
- ^ Chandrasekharan JA, Sharma-Walia N (2015). "Lipoxins: nature's way to resolve inflammation". Journal of Inflammation Research. 8: 181–92. doi:10.2147/JIR.S90380. PMC 4598198. PMID 26457057.
- ^ Romano M, Cianci E, Simiele F, Recchiuti A (2015). "Lipoxins and aspirin-triggered lipoxins in resolution of inflammation". European Journal of Pharmacology. 760: 49–63. doi:10.1016/j.ejphar.2015.03.083. PMID 25895638.
- ^ Clària J, Serhan CN (1995). "Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions". Proc. Natl. Acad. Sci. U.S.A. 92 (21): 9475–9. Bibcode:1995PNAS...92.9475C. doi:10.1073/pnas.92.21.9475. PMC 40824. PMID 7568157.
