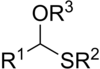

In organosulfur chemistry, thioacetals are the sulfur (thio-) analogues of acetals (R−CH(−OR)2). There are two classes: the less-common monothioacetals, with the formula R−CH(−OR')−SR", and the dithioacetals, with the formula R−CH(−SR')2 (symmetric dithioacetals) or R−CH(−SR')−SR" (asymmetric dithioacetals).[1]
The symmetric dithioacetals are relatively common. They are prepared by condensation of thiols (−SH) or dithiols (two −SH groups) with aldehydes (−CH=O). These reactions proceed via the intermediacy of hemithioacetals (R−CH(−OH)−SR'):
- Thiol addition to give hemithioacetal:
- Thiol addition with loss of water to give dithioacetal:
Such reactions typically employ either a Lewis acid or Brønsted acid as catalyst.
Dithioacetals generated from aldehydes and either 1,2-ethanedithiol or 1,3-propanedithiol are especially common among this class of molecules for use in organic synthesis.[2]
The carbonyl carbon of an aldehyde is electrophilic and therefore susceptible to attack by nucleophiles, whereas the analogous central carbon of a dithioacetal is not electrophilic. As a result, dithioacetals can serve as protective groups for aldehydes.
Far from being unreactive, and in a reaction unlike that of aldehydes, that carbon can be deprotonated to render it nucleophilic:
The inversion of polarity between R'(H)Cδ+=Oδ− and R'CLi(SR)2 is referred to as umpolung. The reaction is commonly performed using the 1,3-dithiane. The lithiated intermediate can be used for various nucleophilic bond-forming reactions, and then the dithioketal hydrolyzed back to its carbonyl form. This overall process, the Corey–Seebach reaction, gives the synthetic equivalent of an acyl anion.
YouTube Encyclopedic
-
1/3Views:49 9744 99912 306
-
Acetals as protecting groups and thioacetals | Organic chemistry | Khan Academy
-
Thioacetals and Raney Nickel Reduction
-
Protecting Groups
Transcription
Voiceover: We've already seen how to form acetals. If we start with an aldehyde or a ketone, and we add an excess of alcohol in an acidic environment we can form our acetal. We talked about ways to increase the formation of your acetal by removing, in this case, water from your reaction to drive the equilibrium to the right. But let's say you increase the concentration of water, so let's say you increase the concentration of this. You would push the equilibrium to the left, and you would hydrolyze your acetal and convert it back into your original aldehyde or ketone and your alcohol. So this can actually be a useful reaction if you want to use an acetal as a protecting group. And so let's look at an example of hydrolyzing an acetal. So, if we have this acetal over here on the left, which is the same one that we made in the previous video, let's see how to analyze the products that we would make by hydrolizing it. So we add HCl as our acid catalyst, and we add an excess of water. And so we're going to get back our original aldehyde and alcohol here. So let's analyze the structure of this. So this carbon right here is the important one so let me go ahead and change color here. So this carbon right here in magenta, if we go back up to here that's this carbon we're talking about here. And so we know that we have a carbon attached to that so that would be this R group right here. And then we also have a hydrogen coming off of that carbon, so that would be this hydrogen right here. And so immediately we can work backwards and see we have an R group we have a hydrogen, we're dealing with an aldehyde right here. Specifically a two-carbon aldehyde, right? So there's two carbons on this, so acetaldehyde is one of our products. So let's go ahead and draw acetaldehyde here, a two-carbon aldehyde. And then our other product, we know is going to be an alcohol. So if we analyze the structure of our acetal, we should be able to figure out what kind of alcohol it is. So if we look at that, that's like this portion right here, which we know came from our alcohol. So all we have to do is add on a hydrogen and then we have the structure of our alcohol product here. So it would be a four-carbon alcohol, so one, two, three, and four, and the exact same thing down here. So our other product would be butanol. And we have two equivalents of it. So that'd be one, two, three, four carbons. So, by looking at our acetal and thinking about hydrolyzing it and thinking about where those portions came from, we can easily come up with the products of this hydrolysis of this acetal. So let's see how we could use this reaction in, if we were trying to synthesize this molecule over here on the right. And so, if we're trying to synthesize this molecule from this molecule, first you might be able to think you could figure it out by looking at the functional groups. Alright, so we have a ketone over here on the left and we want a ketone over here for our product. We also have a carboxylic acid over here to the left, and then we have an alcohol over here on the right. So, if you're thinking about how to complete this kind of transformation, you might think to reduce the carboxylic acid to form your alcohol. And so, you could do that with something like lithium aluminum hydride. So you might think, and you could just add some lithium aluminum hydride here, in the first step. And the second step, add a source of protons to protonate your alkoxide anion to form your alcohol as your product. The only problem with trying to do this in one step is lithium aluminum hydride is also going to reduce your ketone here, to form a secondary alcohol. And so, this will not work. The first thing you have to do is protect the ketone, and then you can use your lithium aluminum hydride. So, we're going to use, we're going to protect our ketone using an acetal, of course. And we're going to react it with ethylene glycol. So if we react our starting compound here with ethylene glycol, and we use an acid catalyst, we're going to form an acetal. Alright, so the ethylene glycol is going to react with the ketone portion of the molecule to form an acetal, specifically a cyclic acetal. Alright, so over here on the left we still have our carboxylic acid. And over here on the right, now we're going to have an oxygen bonded to this carbon, another oxygen bonded to this carbon, and they are, of course, connected. And so, that's our cyclic acetal, which came from this oxygen, this carbon, this carbon, and this oxygen. Right, so that's this oxygen, this carbon, this carbon, and this oxygen. So acetals are stable in basic conditions. And so, now we can add our lithium aluminum hydride, so we add our lithium aluminum hydride here in the first step. And that is going to reduce our carboxylic acid. And so in the second step we can add a source of protons, and we can add some excess water. And so we can protonate the alkoxide anions to form our alcohol product. And also we can add an excess of water in an acidic environment it's going to hydrolize our acetal. So we're also going to hydrolize our cyclic acetal here, and get back our original ketone. And so we've now been able to make our desired product. And so that's one way to use an acetal as a protecting group. Alright, let's look at another type of reaction here. So this one's formation of a thioacetal. So this is completely analogous to the formation of an acetal. So you start off with an aldehyde or a ketone. And this time, instead of using an alcohol you're going to use a thiol, so instead of having an oxygen here, you have a sulfur. So you need acidic conditions, and you can even use something like boron trifluoride here, which can function as a Lewis acid catalyst for this reaction. And you form a thioacetal as your product. So once again you have your R double prime group coming from your thiol, and then you have a sulfur here instead of an oxygen. And then you have two of these to form your thioacetal. And so, one reason you might want to form a thioacetal instead of an acetal, is thioacetals have an additional reaction that they undergo, and we can use it in this transformation. So let's say our goal was to go from the product on the left to the product on the right. And so you can see that we have reduced our ketone here to form this cyclohexene portion. So reducing your ketone. So the first thing we could do, to do this transformation, is to form a thioacetal. So it's going to be, again, analogous to the formation of an acetal. This time we're going to use, instead of a diol, we're going to use something with two SH groups instead here, so a dithiol here. So we can use an acidic environment here. And we're going to form a cyclic thioacetal. So very similar to what we did before. The ring is here, the carboxylic acid is going to be untouched. And instead of an oxygen directly bonded to our ring, we're going to have a sulfur. And the same thing on this side, a sulfur. And then our two carbons. So once again, let's follow those atoms here. So, a sulfur, two carbons, two carbons, and a sulfur. So here's your sulfur, two carbons, two carbons and then another sulfur, so a cyclic thioacetal And then there's actually a reaction for converting this compound into our target compound. And this involves a special kind of nickel, so if we use Raney nickel here. It's a special kind of finely divided nickel that has already adsorbed some hydrogens. And what it's going to do is add some hydrogens to this carbon right here. So we're going to add two hydrogens to this carbon. And you can see that we have reduced the thioacetal and we have formed our desired product. So this is another way to reduce a ketone here, to this alkane portion of the molecule. And so there are other ways to do it, and this just gives you another tool that you can use for synthesis problems.
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "thioacetals". doi:10.1351/goldbook.T06348Error in template * unknown parameter name (GoldBookRef): "title; file"
- ^ P. Stütz And P. A. Stadler "3-alkylated And 3-acylated Indoles From A Common Precursor: 3-benzylindole And 3-benzoylindole" Org. Synth. 1977, 56, 8.doi:10.15227/orgsyn.056.0008




