| |
| Names | |
|---|---|
| IUPAC name
Dimethylmagnesium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6Mg | |
| Molar mass | 54.375 g·mol−1 |
| Density | 0.96 g/cm3 |
| Reacts | |
| Related compounds | |
Related compounds
|
Dibutylmagnesium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethylmagnesium is an organomagnesium compound. It is a white pyrophoric solid.[1][2] Dimethylmagnesium is used in the synthesis of organometallic compounds.
YouTube Encyclopedic
-
1/3Views:5 8624586 715
-
Metallocenes ( CHE)
-
MASTER CADRE CHEMISTRY//ORGANIMATELLIC COMPOUND
-
PART-A BCHET-147 SOLVED ASSIGNMENT 2021-22 // BCHET147 SOLVED ASSIGNMENT //bchet147 #bchet
Transcription
Preparation
Like other dialkylmagnesium compounds, dimethylmagnesium is prepared by adding dioxane to a solution of methylmagnesium halide:[3]
- 2 CH3MgX + 2 dioxane ⇌ (CH3)2Mg + MgX2(μ-dioxane)2↓
In such procedures, the dimethylmagnesium exists as the ether adduct, not the polymer.[4]
Addition of 1,4-dioxane causes precipitation of solid MgX2(μ-dioxane)2, a coordination polymer.[4] This precipitation drives the Schlenk equilibrium toward (CH3)2Mg. Related methods have been applied to other dialkylmagnesium compounds.[3]
Dimethylmagnesium can also be prepared by combining dimethylmercury and magnesium.[5][6]
Properties
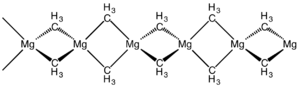
The structure of this compound has been determined by X-ray crystallography. The material is a polymer with the same connectivity as silicon disulfide, featuring tetrahedral magnesium centres, each surrounded by bridging methyl groups. The Mg-C distances are 224 pm.[7] Dimethylberyllium adopts the same structure.[8]
References
- ^ Cope, A. C. (1935). "The Preparation of Dialkylmagnesium Compounds from Grignard Reagents". J. Am. Chem. Soc. 57 (11): 2238–2240. doi:10.1021/ja01314a059.
- ^ Anteunis, M. (1962). "Studies of the Grignard Reaction. II. Kinetics of the Reaction of Dimethylmagnesium with Benzophenone and of Methylmagnesium Bromide-Magnesium Bromide with Pinacolone". J. Org. Chem. 27 (2): 596–598. doi:10.1021/jo01049a060.
- ^ a b Richard A. Andersen, Geoffrey Wilkinson (1979). "Bis[(Trimethylsilyl)Methyl] Magnesium". Inorg. Synth. 19: 262–265. doi:10.1002/9780470132500.ch61.
- ^ a b Fischer, Reinald; Görls, Helmar; Meisinger, Philippe R.; Suxdorf, Regina; Westerhausen, Matthias (2019). "Structure–Solubility Relationship of 1,4‐Dioxane Complexes of Di(hydrocarbyl)magnesium". Chemistry – A European Journal. 25 (55): 12830–12841. doi:10.1002/chem.201903120. PMC 7027550. PMID 31328293.
- ^ Houben-Weyl Methods of Organic Chemistry Vol. XIII/2a, 4th Edition Organometallic Compounds of Group II of the Periodic Table (except mercury) (in German), Georg Thieme Verlag, 2014, p. 215, ISBN 978-3-13-180654-3
- ^ Jane E. Macintyre (1994), Dictionary of Organometallic Compounds (in German), CRC Press, p. 2273, ISBN 978-0-412-43060-2
- ^ Weiss, E. (1964). "Die Kristallstruktur des Dimethylmagnesiums". J. Organomet. Chem. 2 (4): 314–321. doi:10.1016/S0022-328X(00)82217-2.
- ^ Snow, A.I.; Rundle, R.E. (1951). "Structure of Dimethylberyllium". Acta Crystallographica. 4 (4): 348–52. doi:10.1107/S0365110X51001100. hdl:2027/mdp.39015095081207.
